Loss of protein kinase C-δ protects against LPS-induced osteolysis owing to an intrinsic defect in osteoclastic bone resorption
- PMID: 23951014
- PMCID: PMC3738588
- DOI: 10.1371/journal.pone.0070815
Loss of protein kinase C-δ protects against LPS-induced osteolysis owing to an intrinsic defect in osteoclastic bone resorption
Abstract
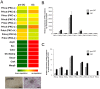
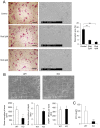
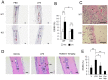
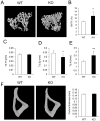
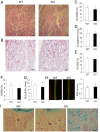
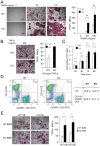
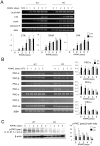
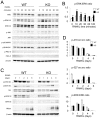
Bone remodeling is intrinsically regulated by cell signaling molecules. The Protein Kinase C (PKC) family of serine/threonine kinases is involved in multiple signaling pathways including cell proliferation, differentiation, apoptosis and osteoclast biology. However, the precise involvement of individual PKC isoforms in the regulation of osteoclast formation and bone homeostasis remains unclear. Here, we identify PKC-δ as the major PKC isoform expressed among all PKCs in osteoclasts; including classical PKCs (-α, -β and -γ), novel PKCs (-δ, -ε, -η and -θ) and atypical PKCs (-ι/λ and -ζ). Interestingly, pharmacological inhibition and genetic ablation of PKC-δ impairs osteoclastic bone resorption in vitro. Moreover, disruption of PKC-δ activity protects against LPS-induced osteolysis in mice, with osteoclasts accumulating on the bone surface failing to resorb bone. Treatment with the PKC-δ inhibitor Rottlerin, blocks LPS-induced bone resorption in mice. Consistently, PKC-δ deficient mice exhibit increased trabeculae bone containing residual cartilage matrix, indicative of an osteoclast-rich osteopetrosis phenotype. Cultured ex vivo osteoclasts derived from PKC-δ null mice exhibit decreased CTX-1 levels and MARKS phosphorylation, with enhanced formation rates. This is accompanied by elevated gene expression levels of cathepsin K and PKC -α, -γ and -ε, as well as altered signaling of pERK and pcSrc416/527 upon RANKL-induction, possibly to compensate for the defects in bone resorption. Collectively, our data indicate that PKC-δ is an intrinsic regulator of osteoclast formation and bone resorption and thus is a potential therapeutic target for pathological osteolysis.
Conflict of interest statement
Figures
Similar articles
-
Alexidine Dihydrochloride Attenuates Osteoclast Formation and Bone Resorption and Protects Against LPS-Induced Osteolysis.J Bone Miner Res. 2016 Mar;31(3):560-72. doi: 10.1002/jbmr.2710. Epub 2016 Jan 6. J Bone Miner Res. 2016. PMID: 26363136
-
PKC-δ deficiency in B cells displays osteopenia accompanied with upregulation of RANKL expression and osteoclast-osteoblast uncoupling.Cell Death Dis. 2020 Sep 16;11(9):762. doi: 10.1038/s41419-020-02947-3. Cell Death Dis. 2020. PMID: 32938907 Free PMC article.
-
Diosmetin inhibits osteoclast formation and differentiation and prevents LPS-induced osteolysis in mice.J Cell Physiol. 2019 Aug;234(8):12701-12713. doi: 10.1002/jcp.27887. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515812
-
PKC delta and epsilon in drug targeting and therapeutics.Recent Pat DNA Gene Seq. 2009;3(2):96-101. doi: 10.2174/187221509788654205. Recent Pat DNA Gene Seq. 2009. PMID: 19519579 Review.
-
Recent advances in osteoclast biology and pathological bone resorption.Histol Histopathol. 2004 Jan;19(1):189-99. doi: 10.14670/HH-19.189. Histol Histopathol. 2004. PMID: 14702187 Review.
Cited by
-
PKCδ deficiency inhibits fetal development and is associated with heart elastic fiber hyperplasia and lung inflammation in adult PKCδ knockout mice.PLoS One. 2021 Jul 1;16(7):e0253912. doi: 10.1371/journal.pone.0253912. eCollection 2021. PLoS One. 2021. PMID: 34197550 Free PMC article.
-
The metabolomics of a protein kinase C delta (PKCδ) knock-out mouse model.Metabolomics. 2022 Nov 13;18(11):92. doi: 10.1007/s11306-022-01949-w. Metabolomics. 2022. PMID: 36371785 Free PMC article.
-
Small molecule inhibitors of osteoarthritis: Current development and future perspective.Front Physiol. 2023 Apr 7;14:1156913. doi: 10.3389/fphys.2023.1156913. eCollection 2023. Front Physiol. 2023. PMID: 37089415 Free PMC article. Review.
-
Osteoclast fusion and bone loss are restricted by interferon inducible guanylate binding proteins.Nat Commun. 2021 Jan 21;12(1):496. doi: 10.1038/s41467-020-20807-8. Nat Commun. 2021. PMID: 33479228 Free PMC article.
-
Andrographolide suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo.Br J Pharmacol. 2014 Feb;171(3):663-75. doi: 10.1111/bph.12463. Br J Pharmacol. 2014. PMID: 24125472 Free PMC article.
References
-
- Kular J, Tickner J, Chim SM, Xu J (2012) An overview of the regulation of bone remodelling at the cellular level. Clinical Biochemistry 45: 863–873. - PubMed
-
- Rodan GA, Martin TJ (2000) Therapeutic Approaches to Bone Diseases. Science 289: 1508–1514. - PubMed
-
- Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289: 1504–1508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

