Protective efficacy of baculovirus dual expression system vaccine expressing Plasmodium falciparum circumsporozoite protein
- PMID: 23951015
- PMCID: PMC3741388
- DOI: 10.1371/journal.pone.0070819
Protective efficacy of baculovirus dual expression system vaccine expressing Plasmodium falciparum circumsporozoite protein
Abstract
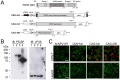
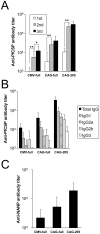
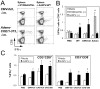
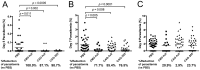
We have previously developed a new malaria vaccine delivery system based on the baculovirus dual expression system (BDES). In this system, expression of malaria antigens is driven by a dual promoter consisting of the baculovirus-derived polyhedrin and mammal-derived cytomegalovirus promoters. To test this system for its potential as a vaccine against human malaria parasites, we investigated immune responses against the newly developed BDES-based Plasmodium falciparum circumsporozoite protein vaccines (BDES-PfCSP) in mice and Rhesus monkeys. Immunization of mice with BDES-PfCSP induced Th1/Th2-mixed type immune responses with high PfCSP-specific antibody (Ab) titers, and provided significant protection against challenge from the bites of mosquitoes infected with a transgenic P. berghei line expressing PfCSP. Next, we evaluated the immunogenicity of the BDES-PfCSP vaccine in a rhesus monkey model. Immunization of BDES-PfCSP elicited high levels of anti-PfCSP Ab responses in individual monkeys. Moreover, the sera from the immunized monkeys remarkably blocked sporozoite invasion of HepG2 cells. Taken together with two animal models, our results indicate that this novel vaccine platform (BDES) has potential clinical application as a vaccine against malaria.
Conflict of interest statement
Figures

Similar articles
-
DAF-shielded baculovirus-vectored vaccine enhances protection against malaria sporozoite challenge in mice.Malar J. 2017 Sep 29;16(1):390. doi: 10.1186/s12936-017-2039-x. Malar J. 2017. PMID: 28962615 Free PMC article.
-
Liver-Directed AAV8 Booster Vaccine Expressing Plasmodium falciparum Antigen Following Adenovirus Vaccine Priming Elicits Sterile Protection in a Murine Model.Front Immunol. 2021 Jun 23;12:612910. doi: 10.3389/fimmu.2021.612910. eCollection 2021. Front Immunol. 2021. PMID: 34248928 Free PMC article.
-
Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine.Malar J. 2013 Apr 22;12:136. doi: 10.1186/1475-2875-12-136. Malar J. 2013. PMID: 23607541 Free PMC article.
-
Prime-boost vectored malaria vaccines: progress and prospects.Hum Vaccin. 2010 Jan;6(1):78-83. doi: 10.4161/hv.6.1.10116. Epub 2010 Jan 18. Hum Vaccin. 2010. PMID: 20061802 Review.
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
Cited by
-
Adenovirus-prime and baculovirus-boost heterologous immunization achieves sterile protection against malaria sporozoite challenge in a murine model.Sci Rep. 2018 Mar 1;8(1):3896. doi: 10.1038/s41598-018-21369-y. Sci Rep. 2018. PMID: 29497047 Free PMC article.
-
Sterile protection and transmission blockade by a multistage anti-malarial vaccine in the pre-clinical study.Front Immunol. 2022 Sep 29;13:1005476. doi: 10.3389/fimmu.2022.1005476. eCollection 2022. Front Immunol. 2022. PMID: 36248835 Free PMC article.
-
Baculovirus capsid display in vaccination schemes: effect of a previous immunity against the vector on the cytotoxic response to delivered antigens.Appl Microbiol Biotechnol. 2018 Dec;102(23):10139-10146. doi: 10.1007/s00253-018-9368-8. Epub 2018 Sep 20. Appl Microbiol Biotechnol. 2018. PMID: 30238142
-
Immunogenicity and transmission-blocking potential of quiescin sulfhydryl oxidase in Plasmodium vivax.Front Cell Infect Microbiol. 2024 Aug 27;14:1451063. doi: 10.3389/fcimb.2024.1451063. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39258252 Free PMC article.
-
Development of a Plasmodium berghei transgenic parasite expressing the full-length Plasmodium vivax circumsporozoite VK247 protein for testing vaccine efficacy in a murine model.Malar J. 2016 Apr 30;15(1):251. doi: 10.1186/s12936-016-1297-3. Malar J. 2016. PMID: 27129682 Free PMC article.
References
-
- (2011) World malaria report 2011. World Health Organization.
-
- Garcon N, Heppner DG, Cohen J (2003) Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines 2: 231–238. - PubMed
-
- Reed SG, Bertholet S, Coler RN, Friede M (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30: 23–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

