Cannabinoid receptor CB2 modulates axon guidance
- PMID: 23951024
- PMCID: PMC3739758
- DOI: 10.1371/journal.pone.0070849
Cannabinoid receptor CB2 modulates axon guidance
Abstract
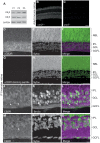
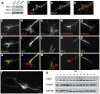
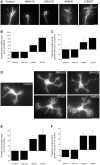
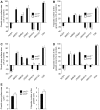
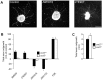
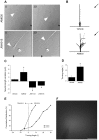
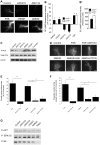
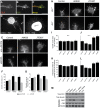
Navigation of retinal projections towards their targets is regulated by guidance molecules and growth cone transduction mechanisms. Here, we present in vitro and in vivo evidences that the cannabinoid receptor 2 (CB2R) is expressed along the retino-thalamic pathway and exerts a modulatory action on axon guidance. These effects are specific to CB2R since no changes were observed in mice where the gene coding for this receptor was altered (cnr2 (-/-)). The CB2R induced morphological changes observed at the growth cone are PKA dependent and require the presence of the netrin-1 receptor, Deleted in Colorectal Cancer. Interfering with endogenous CB2R signalling using pharmacological agents increased retinal axon length and induced aberrant projections. Additionally, cnr2 (-/-) mice showed abnormal eye-specific segregation of retinal projections in the dorsal lateral geniculate nucleus (dLGN) indicating CB2R's implication in retinothalamic development. Overall, this study demonstrates that the contribution of endocannabinoids to brain development is not solely mediated by CB1R, but also involves CB2R.
Conflict of interest statement
Figures

Similar articles
-
Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance.J Neurosci. 2011 Jan 26;31(4):1489-99. doi: 10.1523/JNEUROSCI.4134-09.2011. J Neurosci. 2011. PMID: 21273433 Free PMC article.
-
Cannabinoid type 2 receptors mediate a cell type-specific self-inhibition in cortical neurons.Neuropharmacology. 2018 Sep 1;139:217-225. doi: 10.1016/j.neuropharm.2018.07.020. Epub 2018 Jul 17. Neuropharmacology. 2018. PMID: 30025920
-
Roles of cannabinoid receptors type 1 and 2 on the retinal function of adult mice.Invest Ophthalmol Vis Sci. 2013 Dec 11;54(13):8079-90. doi: 10.1167/iovs.13-12514. Invest Ophthalmol Vis Sci. 2013. PMID: 24255040
-
Cannabinoids and the skeleton: from marijuana to reversal of bone loss.Ann Med. 2009;41(8):560-7. doi: 10.1080/07853890903121025. Ann Med. 2009. PMID: 19634029 Review.
-
Update on the controversial identity of cells expressing cnr2 gene in the nervous system.CNS Neurosci Ther. 2023 Mar;29(3):760-770. doi: 10.1111/cns.13977. Epub 2023 Jan 5. CNS Neurosci Ther. 2023. PMID: 36604187 Free PMC article. Review.
Cited by
-
An endocannabinoid system is present in the mouse olfactory epithelium but does not modulate olfaction.Neuroscience. 2015 Aug 6;300:539-53. doi: 10.1016/j.neuroscience.2015.05.056. Epub 2015 May 30. Neuroscience. 2015. PMID: 26037800 Free PMC article.
-
Interference with the Cannabinoid Receptor CB1R Results in Miswiring of GnRH3 and AgRP1 Axons in Zebrafish Embryos.Int J Mol Sci. 2019 Dec 25;21(1):168. doi: 10.3390/ijms21010168. Int J Mol Sci. 2019. PMID: 31881740 Free PMC article.
-
Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis.Pharmaceutics. 2022 Apr 26;14(5):936. doi: 10.3390/pharmaceutics14050936. Pharmaceutics. 2022. PMID: 35631522 Free PMC article.
-
Receptors of intermediates of carbohydrate metabolism, GPR91 and GPR99, mediate axon growth.PLoS Biol. 2018 May 17;16(5):e2003619. doi: 10.1371/journal.pbio.2003619. eCollection 2018 May. PLoS Biol. 2018. PMID: 29771909 Free PMC article.
-
Cannabinoid Signaling in Auditory Function and Development.Front Mol Neurosci. 2021 May 17;14:678510. doi: 10.3389/fnmol.2021.678510. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34079440 Free PMC article.
References
-
- Salzet M (2000) Invertebrate molecular neuroimmune processes. Brain Res Brain Res Rev 34: 69–79. - PubMed
-
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202. - PubMed
-
- Klein TW, Newton C, Larsen K, Lu L, Perkins I, et al. (2003) The cannabinoid system and immune modulation. J Leukoc Biol 74: 486–496. - PubMed
-
- Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, et al. (1996) The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci U S A 93: 3984–3989. - PMC - PubMed
-
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, et al. (2001) Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology 40: 221–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

