The GPVI-Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding times
- PMID: 23951109
- PMCID: PMC3741334
- DOI: 10.1371/journal.pone.0071193
The GPVI-Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding times
Abstract
Aims: Glycoprotein VI (GPVI) is a key platelet receptor which mediates plaque-induced platelet activation and consecutive atherothrombosis, but GPVI is also involved in platelet-mediated atheroprogression. Therefore, interference in GPVI-mediated platelet activation has the potential to combine short-term and long-term beneficial effects, specificity and safety especially regarding bleeding complications.
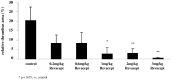
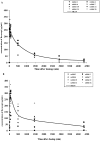
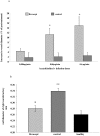
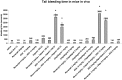
Methods and results: We investigated the effects of the soluble dimeric GPVI receptor fusion protein, Revacept, an antagonist of collagen-mediated platelet activation, in an animal model of atherosclerosis: twenty week old rabbits, which had been fed on a cholesterol-rich diet for 8 weeks, received Revacept (8 mg/kg) or control twice weekly for 4 weeks. Pharmacokinetics indicated a slight accumulation of the drug in the serum after repeated dosing of Revacept for 3 weeks. A significant improvement of endothelial dysfunction after 0.06 and 0.6 µg/min acetylcholine and a significant decrease of vessel wall thickening were found after Revacept treatment. Accordingly, aortic vessel weight was reduced, and plaque sizes, macrophage and T-cell invasion tended to be reduced in histological evaluations. Bleeding time was determined after tail clipping in mice. Revacept alone or in combination with widely used anti-platelet drugs revealed a high safety margin with no prolongation of bleeding times.
Conclusion: Repeated doses of Revacept led to a significant improvement of endothelial dysfunction and vascular morphology in atherosclerotic rabbits. Furthermore, no influence of Revacept on bleeding time alone or in combinations with various anti-platelet drugs was found in mice. Thus, the inhibition of collagen-mediated platelet interaction with the atherosclerotic endothelium by Revacept exerts beneficial effects on morphology and vascular function in vivo and seems to have a wide therapeutic window without influencing the bleeding time.
Conflict of interest statement
Figures






References
-
- Massberg S, Konrad I, Bültmann A, Schulz C, Münch G, et al. (2004) Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J 18(2): 397–399. - PubMed
-
- Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, et al. (2010) A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol 55(11): 1147–1158. - PubMed
-
- Kastrati A, Neumann FJ, Mehilli J, Byrne RA, Iijima R, et al. (2008) Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med 359(7): 688–696. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

