The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica
- PMID: 23951176
- PMCID: PMC3739748
- DOI: 10.1371/journal.pone.0071500
The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica
Abstract
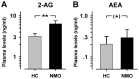
Recurrent myelitis is one of the predominant characteristics in patients with neuromyelitis optica (NMO). While paresis, visual loss, sensory deficits, and bladder dysfunction are well known symptoms in NMO patients, pain has been recognized only recently as another key symptom of the disease. Although spinal cord inflammation is a defining aspect of neuromyelitis, there is an almost complete lack of data on altered somatosensory function, including pain. Therefore, eleven consecutive patients with NMO were investigated regarding the presence and clinical characteristics of pain. All patients were examined clinically as well as by Quantitative Sensory Testing (QST) following the protocol of the German Research Network on Neuropathic Pain (DFNS). Additionally, plasma endocannabinoid levels and signs of chronic stress and depression were determined. Almost all patients (10/11) suffered from NMO-associated neuropathic pain for the last three months, and 8 out of 11 patients indicated relevant pain at the time of examination. Symptoms of neuropathic pain were reported in the vast majority of patients with NMO. Psychological testing revealed signs of marked depression. Compared to age and gender-matched healthy controls, QST revealed pronounced mechanical and thermal sensory loss, strongly correlated to ongoing pain suggesting the presence of deafferentation-induced neuropathic pain. Thermal hyperalgesia correlated to MRI-verified signs of spinal cord lesion. Heat hyperalgesia was highly correlated to the time since last relapse of NMO. Patients with NMO exhibited significant mechanical and thermal dysesthesia, namely dynamic mechanical allodynia and paradoxical heat sensation. Moreover, they presented frequently with either abnormal mechanical hypoalgesia or hyperalgesia, which depended significantly on plasma levels of the endogenous cannabinoid 2-arachidonoylglycerole (2-AG). These data emphasize the high prevalence of neuropathic pain and hyperalgesia in patients with NMO. The degree of mechanical hyperalgesia reflecting central sensitization of nociceptive pathways seems to be controlled by the major brain endocannabinoid 2-AG.
Conflict of interest statement
Figures



Similar articles
-
Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes.Pain. 2010 Sep;150(3):439-450. doi: 10.1016/j.pain.2010.05.002. Pain. 2010. PMID: 20627413
-
Explorative sensory profile evaluation in central neuropathic pain following spinal cord injury.Eur J Pain. 2025 Feb;29(2):e4719. doi: 10.1002/ejp.4719. Epub 2024 Aug 31. Eur J Pain. 2025. PMID: 39215588
-
Bilateral sensory abnormalities in patients with unilateral neuropathic pain; a quantitative sensory testing (QST) study.PLoS One. 2012;7(5):e37524. doi: 10.1371/journal.pone.0037524. Epub 2012 May 22. PLoS One. 2012. PMID: 22629414 Free PMC article.
-
Quantitative Sensory Testing in Spinal Cord Stimulation: A Narrative Review.Neuromodulation. 2024 Aug;27(6):1026-1034. doi: 10.1016/j.neurom.2024.03.005. Epub 2024 Apr 18. Neuromodulation. 2024. PMID: 38639705 Review.
-
Neuromyelitis optica.Curr Opin Neurol. 2007 Jun;20(3):255-60. doi: 10.1097/WCO.0b013e32814f1c6b. Curr Opin Neurol. 2007. PMID: 17495617 Review.
Cited by
-
Quantitative sensory phenotyping in chronic neuropathic pain patients treated with unilateral L4-dorsal root ganglion stimulation.J Transl Med. 2020 Oct 21;18(1):403. doi: 10.1186/s12967-020-02566-8. J Transl Med. 2020. PMID: 33087129 Free PMC article.
-
Clinical measures in chronic neuropathic pain are related to the Kennedy and endocannabinoid pathways.Eur J Clin Invest. 2025 Feb;55(2):e14351. doi: 10.1111/eci.14351. Epub 2024 Nov 15. Eur J Clin Invest. 2025. PMID: 39545479 Free PMC article.
-
Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis.J Neurol. 2023 Jul;270(7):3341-3368. doi: 10.1007/s00415-023-11634-0. Epub 2023 Apr 6. J Neurol. 2023. PMID: 37022481 Free PMC article. Review.
-
Scrambler therapy improves pain in neuromyelitis optica: A randomized controlled trial.Neurology. 2020 May 5;94(18):e1900-e1907. doi: 10.1212/WNL.0000000000009370. Epub 2020 Apr 8. Neurology. 2020. PMID: 32269109 Free PMC article. Clinical Trial.
-
Intrathecal morphine administration reduces postoperative pain and peripheral endocannabinoid levels in total knee arthroplasty patients: a randomized clinical trial.BMC Anesthesiol. 2018 Feb 27;18(1):27. doi: 10.1186/s12871-018-0489-5. BMC Anesthesiol. 2018. PMID: 29486720 Free PMC article. Clinical Trial.
References
-
- Allbutt T (1870) On the opthalmoscopic signs of spinal disease. Lancet: 76–78.
-
- Devic E (1894) Myelite subaiguë compliquée de névrite optique. Bull Med 8: 1033–1034.
-
- Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53: 1107–1114. - PubMed
-
- Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, et al. (2006) Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 63: 964–968. - PubMed
-
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485–1489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

