The multifunctional Ca²⁺/calmodulin-dependent kinase IIδ (CaMKIIδ) regulates arteriogenesis in a mouse model of flow-mediated remodeling
- PMID: 23951185
- PMCID: PMC3738514
- DOI: 10.1371/journal.pone.0071550
The multifunctional Ca²⁺/calmodulin-dependent kinase IIδ (CaMKIIδ) regulates arteriogenesis in a mouse model of flow-mediated remodeling
Abstract
Objective: Sustained hemodynamic stress mediated by high blood flow promotes arteriogenesis, the outward remodeling of existing arteries. Here, we examined whether Ca²⁺/calmodulin-dependent kinase II (CaMKII) regulates arteriogenesis.
Methods and results: Ligation of the left common carotid led to an increase in vessel diameter and perimeter of internal and external elastic lamina in the contralateral, right common carotid. Deletion of CaMKIIδ (CaMKIIδ-/-) abolished this outward remodeling. Carotid ligation increased CaMKII expression and was associated with oxidative activation of CaMKII in the adventitia and endothelium. Remodeling was abrogated in a knock-in model in which oxidative activation of CaMKII is abolished. Early after ligation, matrix metalloproteinase 9 (MMP9) was robustly expressed in the adventitia of right carotid arteries of WT but not CaMKIIδ-/- mice. MMP9 mainly colocalized with adventitial macrophages. In contrast, we did not observe an effect of CaMKIIδ deficiency on other proposed mediators of arteriogenesis such as expression of adhesion molecules or smooth muscle proliferation. Transplantation of WT bone marrow into CaMKIIδ-/- mice normalized flow-mediated remodeling.
Conclusion: CaMKIIδ is activated by oxidation under high blood flow conditions and is required for flow-mediated remodeling through a mechanism that includes increased MMP9 expression in bone marrow-derived cells invading the arterial wall.
Conflict of interest statement
Figures

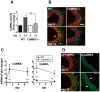
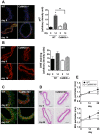
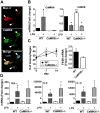
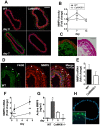

Similar articles
-
Oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis.Vascul Pharmacol. 2014 Feb;60(2):75-83. doi: 10.1016/j.vph.2014.01.001. Epub 2014 Jan 10. Vascul Pharmacol. 2014. PMID: 24418021 Free PMC article.
-
The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9.Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1953-64. doi: 10.1152/ajpheart.00978.2011. Epub 2012 Mar 16. Am J Physiol Heart Circ Physiol. 2012. PMID: 22427508 Free PMC article.
-
The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21.J Biol Chem. 2011 Mar 11;286(10):7990-7999. doi: 10.1074/jbc.M110.163006. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193397 Free PMC article.
-
Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes?Recent Prog Horm Res. 2004;59:141-68. doi: 10.1210/rp.59.1.141. Recent Prog Horm Res. 2004. PMID: 14749501 Review.
-
Ca2+/calmodulin-dependent protein kinase II function in vascular remodelling.J Physiol. 2012 Mar 15;590(6):1349-56. doi: 10.1113/jphysiol.2011.222232. Epub 2011 Nov 28. J Physiol. 2012. PMID: 22124148 Free PMC article. Review.
Cited by
-
Role of CaMKII in Ang-II-dependent small artery remodeling.Vascul Pharmacol. 2016 Dec;87:172-179. doi: 10.1016/j.vph.2016.09.007. Epub 2016 Sep 20. Vascul Pharmacol. 2016. PMID: 27658984 Free PMC article.
-
CaMKII as a Therapeutic Target in Cardiovascular Disease.Annu Rev Pharmacol Toxicol. 2023 Jan 20;63:249-272. doi: 10.1146/annurev-pharmtox-051421-111814. Epub 2022 Aug 16. Annu Rev Pharmacol Toxicol. 2023. PMID: 35973713 Free PMC article. Review.
-
Ca2+/calmodulin-dependent protein kinase II-γ (CaMKIIγ) negatively regulates vascular smooth muscle cell proliferation and vascular remodeling.FASEB J. 2016 Mar;30(3):1051-64. doi: 10.1096/fj.15-279158. Epub 2015 Nov 13. FASEB J. 2016. PMID: 26567004 Free PMC article.
-
Role of CaMKII in diabetes induced vascular injury and its interaction with anti-diabetes therapy.Rev Endocr Metab Disord. 2024 Apr;25(2):369-382. doi: 10.1007/s11154-023-09855-9. Epub 2023 Dec 8. Rev Endocr Metab Disord. 2024. PMID: 38064002 Free PMC article. Review.
-
Oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis.Vascul Pharmacol. 2014 Feb;60(2):75-83. doi: 10.1016/j.vph.2014.01.001. Epub 2014 Jan 10. Vascul Pharmacol. 2014. PMID: 24418021 Free PMC article.
References
-
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, et al. (2001) Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286: 1317–1324. - PubMed
-
- Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodeling. N Engl J Med 330: 1431–1438. - PubMed
-
- van Royen N, Piek JJ, Schaper W, Fulton WF (2009) A critical review of clinical arteriogenesis research. J Am Coll Cardiol 55: 17–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL 070250/HL/NHLBI NIH HHS/United States
- R01 HL108932/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- I01 BX000163/BX/BLRD VA/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- R01 AA 019568/AA/NIAAA NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- R01 AA019568/AA/NIAAA NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R01 HL 108932/HL/NHLBI NIH HHS/United States
- R01 HL 079031/HL/NHLBI NIH HHS/United States
- T32 HL007121/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

