Reconstructing the Qo site of Plasmodium falciparum bc 1 complex in the yeast enzyme
- PMID: 23951230
- PMCID: PMC3741170
- DOI: 10.1371/journal.pone.0071726
Reconstructing the Qo site of Plasmodium falciparum bc 1 complex in the yeast enzyme
Abstract
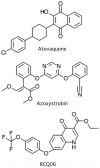
The bc 1 complex of the mitochondrial respiratory chain is essential for Plasmodium falciparum proliferation, the causative agent of human malaria. Therefore, this enzyme is an attractive target for antimalarials. However, biochemical investigations of the parasite enzyme needed for the study of new drugs are challenging. In order to facilitate the study of new compounds targeting the enzyme, we are modifying the inhibitor binding sites of the yeast Saccharomyces cerevisiae to generate a complex that mimics the P. falciparum enzyme. In this study we focused on its Qo pocket, the site of atovaquone binding which is a leading antimalarial drug used in treatment and causal prophylaxis. We constructed and studied a series of mutants with modified Qo sites where yeast residues have been replaced by P. falciparum equivalents, or, for comparison, by human equivalents. Mitochondria were prepared from the yeast Plasmodium-like and human-like Qo mutants. We measured the bc 1 complex sensitivity to atovaquone, azoxystrobin, a Qo site targeting fungicide active against P. falciparum and RCQ06, a quinolone-derivative inhibitor of P. falciparum bc 1 complex.The data obtained highlighted variations in the Qo site that could explain the differences in inhibitor sensitivity between yeast, plasmodial and human enzymes. We showed that the yeast Plasmodium-like Qo mutants could be useful and easy-to-use tools for the study of that class of antimalarials.
Conflict of interest statement
Figures

Similar articles
-
Subtle changes in endochin-like quinolone structure alter the site of inhibition within the cytochrome bc1 complex of Plasmodium falciparum.Antimicrob Agents Chemother. 2015 Apr;59(4):1977-82. doi: 10.1128/AAC.04149-14. Epub 2015 Jan 20. Antimicrob Agents Chemother. 2015. PMID: 25605352 Free PMC article.
-
Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex.Parasitol Int. 2015 Jun;64(3):295-300. doi: 10.1016/j.parint.2014.09.011. Epub 2014 Sep 28. Parasitol Int. 2015. PMID: 25264100
-
Saccharomyces cerevisiae-based mutational analysis of the bc1 complex Qo site residue 279 to study the trade-off between atovaquone resistance and function.Antimicrob Agents Chemother. 2015 Jul;59(7):4053-8. doi: 10.1128/AAC.00710-15. Epub 2015 Apr 27. Antimicrob Agents Chemother. 2015. PMID: 25918152 Free PMC article.
-
Modeling the molecular basis of atovaquone resistance in parasites and pathogenic fungi.Trends Parasitol. 2007 Oct;23(10):494-501. doi: 10.1016/j.pt.2007.08.004. Epub 2007 Sep 7. Trends Parasitol. 2007. PMID: 17826334 Review.
-
Inhibiting Plasmodium cytochrome bc1: a complex issue.Curr Opin Chem Biol. 2010 Aug;14(4):440-6. doi: 10.1016/j.cbpa.2010.05.005. Curr Opin Chem Biol. 2010. PMID: 20570550 Review.
Cited by
-
Design, synthesis, and biological evaluation of multiple targeting antimalarials.Acta Pharm Sin B. 2021 Sep;11(9):2900-2913. doi: 10.1016/j.apsb.2021.05.008. Epub 2021 May 20. Acta Pharm Sin B. 2021. PMID: 34589403 Free PMC article.
-
Screening the Medicines for Malaria Venture Pathogen Box against piroplasm parasites.Int J Parasitol Drugs Drug Resist. 2019 Aug;10:84-90. doi: 10.1016/j.ijpddr.2019.06.004. Epub 2019 Jun 19. Int J Parasitol Drugs Drug Resist. 2019. PMID: 31254719 Free PMC article.
-
Subtle changes in endochin-like quinolone structure alter the site of inhibition within the cytochrome bc1 complex of Plasmodium falciparum.Antimicrob Agents Chemother. 2015 Apr;59(4):1977-82. doi: 10.1128/AAC.04149-14. Epub 2015 Jan 20. Antimicrob Agents Chemother. 2015. PMID: 25605352 Free PMC article.
-
Human Mitochondrial Cytochrome b Variants Studied in Yeast: Not All Are Silent Polymorphisms.Hum Mutat. 2016 Sep;37(9):933-41. doi: 10.1002/humu.23024. Epub 2016 Jun 27. Hum Mutat. 2016. PMID: 27291790 Free PMC article.
-
Antiplasmodial potential of isolated xanthones from Mesua ferrea Linn. roots: an in vitro and in silico molecular docking and pharmacokinetics study.BMC Complement Med Ther. 2024 Jul 25;24(1):282. doi: 10.1186/s12906-024-04580-5. BMC Complement Med Ther. 2024. PMID: 39054443 Free PMC article.
References
-
- Painter HJ, Morrisey JM, Mather MW, Vaidya AB (2007) Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum . Nature 446: 88–91. - PubMed
-
- Musset L, Bouchaud O, Matheron S, Massias L, Le Bras J (2006) Clinical atovaquone-proguanil resistance of Plasmodium falciparum associated with cytochrome b codon 268 mutations. Microbes Infect 8: 2599–2604. - PubMed
-
- Musset L, Le Bras J, Clain J (2007) Parallel evolution of adaptive mutations in Plasmodium falciparum mitochondrial DNA during atovaquone-proguanil treatment. Mol Biol Evol 24: 1582–1585. - PubMed
-
- Barton V, Fisher N, Biagini GA, Ward SA, O'Neill P (2010) Inhibiting Plasmodium cytochrome bc 1: a complex issue. Curr Opin Chem Biol 14: 1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

