Influence of parasite load on renal function in mice acutely infected with Trypanosoma cruzi
- PMID: 23951243
- PMCID: PMC3741127
- DOI: 10.1371/journal.pone.0071772
Influence of parasite load on renal function in mice acutely infected with Trypanosoma cruzi
Abstract
Background: Chagas disease is a neglected tropical disease caused by Trypanosoma cruzi. Despite the vast number of studies evaluating the pathophysiological mechanisms of the disease, the influence of parasite burden on kidney lesions remains unclear. Thus, the main goal of this work was to evaluate the effect of T. cruzi infection on renal function and determine whether there was a correlation between parasite load and renal injury using an acute experimental model of the disease.
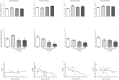
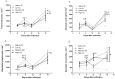
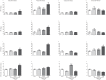
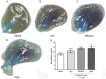
Methodology/principal findings: Low, medium and high parasite loads were generated by infecting C57BL/6 mice with 300 (low), 3,000 (medium) or 30,000 (high) numbers of "Y" strain trypomastigotes. We found that mice infected with T. cruzi trypomastigotes show increased renal injury. The infection resulted in reduced urinary excretion and creatinine clearance. We also observed a marked elevation in the ratio of urine volume to kidney and body weight, blood urea nitrogen, chloride ion, nitric oxide, pro- and anti-inflammatory cytokines and the number of leukocytes in the blood and/or renal tissues of infected mice. Additionally, we observed the presence of the parasite in the cortical/medullary and peri-renal region, an increase of inflammatory infiltrate and of vascular permeability of the kidney. Overall, most renal changes occurred mainly in animals infected with high parasitic loads.
Conclusions/significance: These data demonstrate that T. cruzi impairs kidney function, and this impairment is more evident in mice infected with high parasitic loads. Moreover, these data suggest that, in addition to the extensively studied cardiovascular effects, renal injury should be regarded as an important indicator for better understanding the pan-infectivity of the parasite and consequently for understanding the disease in experimental models.
Conflict of interest statement
Figures



Similar articles
-
Inflammatory responses and intestinal injury development during acute Trypanosoma cruzi infection are associated with the parasite load.Parasit Vectors. 2015 Apr 3;8:206. doi: 10.1186/s13071-015-0811-8. Parasit Vectors. 2015. PMID: 25889515 Free PMC article.
-
Differential susceptibility to acute Trypanosoma cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities.Clin Exp Immunol. 2002 Jun;128(3):421-8. doi: 10.1046/j.1365-2249.2002.01874.x. Clin Exp Immunol. 2002. PMID: 12067296 Free PMC article.
-
Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain.Int J Parasitol Drugs Drug Resist. 2015 Dec 12;6(1):12-22. doi: 10.1016/j.ijpddr.2015.12.001. eCollection 2016 Apr. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26862474 Free PMC article.
-
Mitochondrial and satellite real time-PCR for detecting T. cruzi DTU II strain in blood and organs of experimentally infected mice presenting different levels of parasite load.Exp Parasitol. 2019 May;200:13-15. doi: 10.1016/j.exppara.2019.03.007. Epub 2019 Mar 20. Exp Parasitol. 2019. PMID: 30904696 Review.
-
Bioactive lipids in Trypanosoma cruzi infection.Adv Parasitol. 2011;76:1-31. doi: 10.1016/B978-0-12-385895-5.00001-3. Adv Parasitol. 2011. PMID: 21884885 Free PMC article. Review.
Cited by
-
Consumption of Galactose by Trypanosoma cruzi Epimastigotes Generates Resistance against Oxidative Stress.Pathogens. 2022 Oct 11;11(10):1174. doi: 10.3390/pathogens11101174. Pathogens. 2022. PMID: 36297231 Free PMC article.
-
Predicting Blood Parasite Load and Influence of Expression of iNOS on the Effect Size of Clinical Laboratory Parameters in Acute Trypanosoma cruzi Infection With Different Inoculum Concentrations in C57BL/6 Mice.Front Immunol. 2022 Mar 18;13:850037. doi: 10.3389/fimmu.2022.850037. eCollection 2022. Front Immunol. 2022. PMID: 35371021 Free PMC article.
-
Gastrointestinal Motility, Mucosal Mast Cell, and Intestinal Histology in Rats: Effect of Prednisone.Biomed Res Int. 2017;2017:4637621. doi: 10.1155/2017/4637621. Epub 2017 Sep 19. Biomed Res Int. 2017. PMID: 29057260 Free PMC article.
-
Lower urinary tract dysfunction in chronic Chagas disease: clinical and urodynamic presentation.World J Urol. 2019 Jul;37(7):1395-1402. doi: 10.1007/s00345-018-2512-3. Epub 2018 Oct 9. World J Urol. 2019. PMID: 30302592 Free PMC article.
-
Efficacy of benznidazole delivery during Chagas disease nanotherapy is dependent on the nanocarrier morphology.Biomaterials. 2025 Nov;322:123358. doi: 10.1016/j.biomaterials.2025.123358. Epub 2025 Apr 22. Biomaterials. 2025. PMID: 40318604
References
-
- Kute VB, Goswami JG, Vanikar AV, Shah PR, Gumber MR, et al.. (2011) Unusual presentation of Plasmodium vivax: a neglected human malaria parasite. Parasitol Res DOI –––10.1007/s00436–011–2776–7 - DOI - PubMed
-
- Gurjar M, Saigal S, Baronia AK, Azim A, Poddar B, et al. (2011) Clinical manifestations of co-infection with malaria and leptospirosis. Trop Doct 41: 175–178. - PubMed
-
- Chagas C (1909) Nova tripanozomiase humana: estudos sobre a morphologia e o ciclo evolutivo do Schizotripanum cruzi, agente etiológico da nova entidade mórbida do homem. Mem Inst Oswaldo Cruz1: 159–218.
-
- Dias JC (2007) Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusional Chagas disease. Historical aspects, present situation and perspectives. Mem Inst Oswaldo Cruz 102: 11–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

