Metformin protects rat hepatocytes against bile acid-induced apoptosis
- PMID: 23951244
- PMCID: PMC3741108
- DOI: 10.1371/journal.pone.0071773
Metformin protects rat hepatocytes against bile acid-induced apoptosis
Abstract
Background: Metformin is used in the treatment of Diabetes Mellitus type II and improves liver function in patients with non-alcoholic fatty liver disease (NAFLD). Metformin activates AMP-activated protein kinase (AMPK), the cellular energy sensor that is sensitive to changes in the AMP/ATP-ratio. AMPK is an inhibitor of mammalian target of rapamycin (mTOR). Both AMPK and mTOR are able to modulate cell death.
Aim: To evaluate the effects of metformin on hepatocyte cell death.
Methods: Apoptotic cell death was induced in primary rat hepatocytes using either the bile acid glycochenodeoxycholic acid (GCDCA) or TNFα in combination with actinomycin D (actD). AMPK, mTOR and phosphoinositide-3 kinase (PI3K)/Akt were inhibited using pharmacological inhibitors. Apoptosis and necrosis were quantified by caspase activation, acridine orange staining and Sytox green staining respectively.
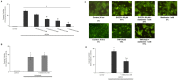
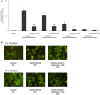
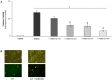
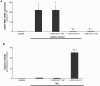
Results: Metformin dose-dependently reduces GCDCA-induced apoptosis, even when added 2 hours after GCDCA, without increasing necrotic cell death. Metformin does not protect against TNFα/ActD-induced apoptosis. The protective effect of metformin is dependent on an intact PI3-kinase/Akt pathway, but does not require AMPK/mTOR-signaling. Metformin does not inhibit NF-κB activation.
Conclusion: Metformin protects against bile acid-induced apoptosis and could be considered in the treatment of chronic liver diseases accompanied by inflammation.
Conflict of interest statement
Figures


Similar articles
-
The active metabolite of leflunomide, A77 1726, protects rat hepatocytes against bile acid-induced apoptosis.J Hepatol. 2008 Nov;49(5):799-809. doi: 10.1016/j.jhep.2008.07.019. Epub 2008 Aug 22. J Hepatol. 2008. PMID: 18809221
-
Pertussis toxin, an inhibitor of G(αi) PCR, inhibits bile acid- and cytokine-induced apoptosis in primary rat hepatocytes.PLoS One. 2012;7(8):e43156. doi: 10.1371/journal.pone.0043156. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900098 Free PMC article.
-
Angiotensin II protects primary rat hepatocytes against bile salt-induced apoptosis.PLoS One. 2012;7(12):e52647. doi: 10.1371/journal.pone.0052647. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300732 Free PMC article.
-
Metformin in cardiovascular diabetology: a focused review of its impact on endothelial function.Theranostics. 2021 Sep 9;11(19):9376-9396. doi: 10.7150/thno.64706. eCollection 2021. Theranostics. 2021. PMID: 34646376 Free PMC article. Review.
-
The role of AMPK/mTOR signaling pathway in anticancer activity of metformin.Physiol Res. 2021 Aug 31;70(4):501-508. doi: 10.33549/physiolres.934618. Epub 2021 Jun 1. Physiol Res. 2021. PMID: 34062070 Free PMC article. Review.
Cited by
-
Metformin protects primary rat hepatocytes against oxidative stress-induced apoptosis.Pharmacol Res Perspect. 2015 Mar;3(2):e00125. doi: 10.1002/prp2.125. Epub 2015 Mar 18. Pharmacol Res Perspect. 2015. PMID: 26038701 Free PMC article.
-
Metformin enhances LDL-cholesterol uptake by suppressing the expression of the pro-protein convertase subtilisin/kexin type 9 (PCSK9) in liver cells.Endocrine. 2022 Jun;76(3):543-557. doi: 10.1007/s12020-022-03022-x. Epub 2022 Mar 2. Endocrine. 2022. PMID: 35237909
-
Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids.J Lipid Res. 2017 Jul;58(7):1399-1416. doi: 10.1194/jlr.M075713. Epub 2017 May 22. J Lipid Res. 2017. PMID: 28533304 Free PMC article.
-
Radioprotective effect of the anti-diabetic drug metformin.PLoS One. 2024 Jul 23;19(7):e0307598. doi: 10.1371/journal.pone.0307598. eCollection 2024. PLoS One. 2024. PMID: 39042641 Free PMC article.
-
Metformin Preconditioning Improves Hepatobiliary Function and Reduces Injury in a Rat Model of Normothermic Machine Perfusion and Orthotopic Transplantation.Transplantation. 2020 Sep;104(9):e271-e280. doi: 10.1097/TP.0000000000003216. Transplantation. 2020. PMID: 32150043 Free PMC article.
References
-
- Klein J, Westphal S, Kraus D, Meier B, Perwitz N, et al. (2004) Metformin inhibits leptin secretion via a mitogen-activated protein kinase signalling pathway in brown adipocytes. J Endocrinol 183: 299–307. - PubMed
-
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, et al. (2000) Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nature Medicine 6: 998–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

