Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation
- PMID: 23951248
- PMCID: PMC3741203
- DOI: 10.1371/journal.pone.0071814
Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation
Abstract
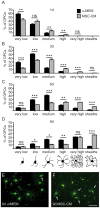
Oligodendroglial progenitor/precursor cells (OPCs) represent the main cellular source for the generation of new myelinating oligodendrocytes in the adult central nervous system (CNS). In demyelinating diseases such as multiple sclerosis (MS) myelin repair activities based on recruitment, activation and differentiation of resident OPCs can be observed. However, the overall degree of successful remyelination is limited and the existence of an MS-derived anti-oligodendrogenic milieu prevents OPCs from contributing to myelin repair. It is therefore of considerable interest to understand oligodendroglial homeostasis and maturation processes in order to enable the development of remyelination therapies. Mesenchymal stem cells (MSC) have been shown to exert positive immunomodulatory effects, reduce demyelination, increase neuroprotection and to promote adult neural stem cell differentiation towards the oligodendroglial lineage. We here addressed whether MSC secreted factors can boost the OPC's oligodendrogenic capacity in a myelin non-permissive environment. To this end, we analyzed cellular morphologies, expression and regulation of key factors involved in oligodendroglial fate and maturation of primary rat cells upon incubation with MSC-conditioned medium. This demonstrated that MSC-derived soluble factors promote and accelerate oligodendroglial differentiation, even under astrocytic endorsing conditions. Accelerated maturation resulted in elevated levels of myelin expression, reduced glial fibrillary acidic protein expression and was accompanied by downregulation of prominent inhibitory differentiation factors such as Id2 and Id4. We thus conclude that apart from their suggested application as potential anti-inflammatory and immunomodulatory MS treatment, these cells might also be exploited to support endogenous myelin repair activities.
Conflict of interest statement
Figures







References
-
- Kremer D, Aktas O, Hartung HP, Küry P (2011) The complex world of oligodendroglial differentiation inhibitors. Ann Neurol 69: 602–618. - PubMed
-
- Kotter MR, Stadelmann C, Hartung HP (2011) Enhancing remyelination in disease–can we wrap it up? Brain 134: 1882–1900. - PubMed
-
- Aktas O, Kieseier B, Hartung HP (2010) Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci 33: 140–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

