Optimisation of recombinant production of active human cardiac SERCA2a ATPase
- PMID: 23951256
- PMCID: PMC3741278
- DOI: 10.1371/journal.pone.0071842
Optimisation of recombinant production of active human cardiac SERCA2a ATPase
Abstract
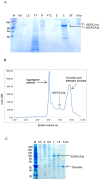
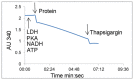
Methods for recombinant production of eukaryotic membrane proteins, yielding sufficient quantity and quality of protein for structural biology, remain a challenge. We describe here, expression and purification optimisation of the human SERCA2a cardiac isoform of Ca(2+) translocating ATPase, using Saccharomyces cerevisiae as the heterologous expression system of choice. Two different expression vectors were utilised, allowing expression of C-terminal fusion proteins with a biotinylation domain or a GFP- His8 tag. Solubilised membrane fractions containing the protein of interest were purified onto Streptavidin-Sepharose, Ni-NTA or Talon resin, depending on the fusion tag present. Biotinylated protein was detected using specific antibody directed against SERCA2 and, advantageously, GFP-His8 fusion protein was easily traced during the purification steps using in-gel fluorescence. Importantly, talon resin affinity purification proved more specific than Ni-NTA resin for the GFP-His8 tagged protein, providing better separation of oligomers present, during size exclusion chromatography. The optimised method for expression and purification of human cardiac SERCA2a reported herein, yields purified protein (> 90%) that displays a calcium-dependent thapsigargin-sensitive activity and is suitable for further biophysical, structural and physiological studies. This work provides support for the use of Saccharomyces cerevisiae as a suitable expression system for recombinant production of multi-domain eukaryotic membrane proteins.
Conflict of interest statement
Figures




Similar articles
-
Heterologous expression and affinity purification of eukaryotic membrane proteins in view of functional and structural studies: The example of the sarcoplasmic reticulum Ca(2+)-ATPase.Methods Mol Biol. 2010;601:247-67. doi: 10.1007/978-1-60761-344-2_15. Methods Mol Biol. 2010. PMID: 20099150
-
Expression in yeast and purification of a membrane protein, SERCA1a, using a biotinylated acceptor domain.Protein Expr Purif. 2006 Jul;48(1):32-42. doi: 10.1016/j.pep.2006.03.001. Epub 2006 Mar 24. Protein Expr Purif. 2006. PMID: 16603381
-
Saccharomyces cerevisiae-based platform for rapid production and evaluation of eukaryotic nutrient transporters and transceptors for biochemical studies and crystallography.PLoS One. 2013 Oct 4;8(10):e76851. doi: 10.1371/journal.pone.0076851. eCollection 2013. PLoS One. 2013. PMID: 24124599 Free PMC article.
-
The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases.Nat Clin Pract Cardiovasc Med. 2008 Sep;5(9):554-65. doi: 10.1038/ncpcardio1301. Epub 2008 Jul 29. Nat Clin Pract Cardiovasc Med. 2008. PMID: 18665137 Review.
-
Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation.Cardiovasc Res. 2003 Jan;57(1):20-7. doi: 10.1016/s0008-6363(02)00694-6. Cardiovasc Res. 2003. PMID: 12504810 Review.
Cited by
-
eGFP as an All-in-One Tag for Purification of Membrane Proteins.Methods Mol Biol. 2023;2652:171-186. doi: 10.1007/978-1-0716-3147-8_9. Methods Mol Biol. 2023. PMID: 37093475
-
Isolation of the Sarcoplasmic Reticulum Ca2+-ATPase from Rabbit Fast-Twitch Muscle.Methods Protoc. 2023 Oct 19;6(5):102. doi: 10.3390/mps6050102. Methods Protoc. 2023. PMID: 37888034 Free PMC article.
-
Expression of Rift Valley fever virus N-protein in Nicotiana benthamiana for use as a diagnostic antigen.BMC Biotechnol. 2018 Dec 11;18(1):77. doi: 10.1186/s12896-018-0489-z. BMC Biotechnol. 2018. PMID: 30537953 Free PMC article.
References
-
- Toyoshima C (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793: 941-946. doi:10.1016/j.bbamcr.2008.10.008. PubMed: 19010358. - DOI - PubMed
-
- Møller JV, Olesen C, Winther A-ML, Nissen P (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys 43: 501-566. doi:10.1017/S003358351000017X. PubMed: 20809990. - DOI - PubMed
-
- Misquitta CM, Mack DP, Grover AK (1999) Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium 25: 277-290. doi:10.1054/ceca.1999.0032. PubMed: 10456225. - DOI - PubMed
-
- Misquitta CM, Sing A, Grover AK (1999) Control of sarcoplasmic/endoplasmic-reticulum Ca2+ pump expression in cardiac and smooth muscle. Biochem J 338(Pt 1): 167-173. doi:10.1042/0264-6021:3380167. PubMed: 9931313. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

