Stock market returns and clinical trial results of investigational compounds: an event study analysis of large biopharmaceutical companies
- PMID: 23951273
- PMCID: PMC3737210
- DOI: 10.1371/journal.pone.0071966
Stock market returns and clinical trial results of investigational compounds: an event study analysis of large biopharmaceutical companies
Abstract
Background: For biopharmaceutical companies, investments in research and development are risky, and the results from clinical trials are key inflection points in the process. Few studies have explored how and to what extent the public equity market values clinical trial results.
Methods: Our study dataset matched announcements of clinical trial results for investigational compounds from January 2011 to May 2013 with daily stock market returns of large United States-listed pharmaceutical and biotechnology companies. Event study methodology was used to examine the relationship between clinical research events and changes in stock returns.
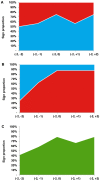
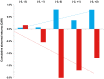
Results: We identified public announcements for clinical trials of 24 investigational compounds, including 16 (67%) positive and 8 (33%) negative events. The majority of announcements were for Phase 3 clinical trials (N = 13, 54%), and for oncologic (N = 7, 29%) and neurologic (N = 6, 24%) indications. The median cumulative abnormal returns on the day of the announcement were 0.8% (95% confidence interval [CI]: -2.3, 13.4%; P = 0.02) for positive events and -2.0% (95% CI: -9.1, 0.7%; P = 0.04) for negative events, with statistically significant differences from zero. In the day immediately following the announcement, firms with positive events were associated with stock price corrections, with median cumulative abnormal returns falling to 0.4% (95% CI: -3.8, 12.3%; P = 0.33). For firms with negative announcements, the median cumulative abnormal returns were -1.7% (95% CI: -9.5, 1.0%; P = 0.03), and remained significantly negative over the two day event window. The magnitude of abnormal returns did not differ statistically by indication, by trial phase, or between biotechnology and pharmaceutical firms.
Conclusions: The release of clinical trial results is an economically significant event and has meaningful effects on market value for large biopharmaceutical companies. Stock return underperformance due to negative events is greater in magnitude and persists longer than abnormal returns due to positive events, suggesting asymmetric market reactions.
Conflict of interest statement
Figures

References
-
- nnell JW, Blanckley A, Boldon H, Warrington B (2012) Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 11: 191–200. - PubMed
-
- Getz KA, Wenger J, Campo RA, Seguine ES, Kaitin KI (2008) Assessing the impact of protocol design changes on clinical trial performance. Am J Ther 15: 450–457. - PubMed
-
- Munos B (2009) Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 8: 959–968. - PubMed
-
- Pammolli F, Magazzini L, Riccaboni M (2011) The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 10: 428–438. - PubMed
-
- DiMasi JA, Feldman L, Seckler A, Wilson A (2010) Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther 87: 272–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

