Us3 kinase encoded by herpes simplex virus 1 mediates downregulation of cell surface major histocompatibility complex class I and evasion of CD8+ T cells
- PMID: 23951282
- PMCID: PMC3741198
- DOI: 10.1371/journal.pone.0072050
Us3 kinase encoded by herpes simplex virus 1 mediates downregulation of cell surface major histocompatibility complex class I and evasion of CD8+ T cells
Abstract
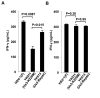
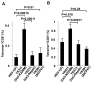
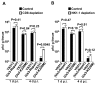
Detection and elimination of virus-infected cells by CD8(+) cytotoxic T lymphocytes (CTLs) depends on recognition of virus-derived peptides presented by major histocompatibility complex class I (MHC-I) molecules on the surface of infected cells. In the present study, we showed that inactivation of the activity of viral kinase Us3 encoded by herpes simplex virus 1 (HSV-1), the etiologic agent of several human diseases and a member of the alphaherpesvirinae, significantly increased cell surface expression of MHC-I, thereby augmenting CTL recognition of infected cells in vitro. Overexpression of Us3 by itself had no effect on cell surface expression of MHC-I and Us3 was not able to phosphorylate MHC-I in vitro, suggesting that Us3 indirectly downregulated cell surface expression of MHC-I in infected cells. We also showed that inactivation of Us3 kinase activity induced significantly more HSV-1-specific CD8(+) T cells in mice. Interestingly, depletion of CD8(+) T cells in mice significantly increased replication of a recombinant virus encoding a kinase-dead mutant of Us3, but had no effect on replication of a recombinant virus in which the kinase-dead mutation was repaired. These results indicated that Us3 kinase activity is required for efficient downregulation of cell surface expression of MHC-I and mediates evasion of HSV-1-specific CD8(+) T cells. Our results also raised the possibility that evasion of HSV-1-specific CD8(+) T cells by HSV-1 Us3-mediated inhibition of MHC-I antigen presentation might in part contribute to viral replication in vivo.
Conflict of interest statement
Figures
Similar articles
-
Impact of the changes in substrate specificity of herpes simplex virus 1 protein kinase Us3 on viral infection in vitro and in vivo.J Virol. 2025 Jul 22;99(7):e0040025. doi: 10.1128/jvi.00400-25. Epub 2025 Jun 30. J Virol. 2025. PMID: 40586577 Free PMC article.
-
Phosphorylation of a herpes simplex virus 1 dUTPase by a viral protein kinase, Us3, dictates viral pathogenicity in the central nervous system but not at the periphery.J Virol. 2014 Mar;88(5):2775-85. doi: 10.1128/JVI.03300-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352467 Free PMC article.
-
Phosphoregulation of a Conserved Herpesvirus Tegument Protein by a Virally Encoded Protein Kinase in Viral Pathogenicity and Potential Linkage between Its Evolution and Viral Phylogeny.J Virol. 2020 Aug 31;94(18):e01055-20. doi: 10.1128/JVI.01055-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32611749 Free PMC article.
-
Us3 Protein Kinase Encoded by HSV: The Precise Function and Mechanism on Viral Life Cycle.Adv Exp Med Biol. 2018;1045:45-62. doi: 10.1007/978-981-10-7230-7_3. Adv Exp Med Biol. 2018. PMID: 29896662 Review.
-
Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus.Curr Top Microbiol Immunol. 2002;269:101-15. doi: 10.1007/978-3-642-59421-2_7. Curr Top Microbiol Immunol. 2002. PMID: 12224504 Review.
Cited by
-
Equine Herpesvirus 1 Multiply Inserted Transmembrane Protein pUL43 Cooperates with pUL56 in Downregulation of Cell Surface Major Histocompatibility Complex Class I.J Virol. 2015 Jun;89(12):6251-63. doi: 10.1128/JVI.00032-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833055 Free PMC article.
-
The Roseoloviruses Downregulate the Protein Tyrosine Phosphatase PTPRC (CD45).J Virol. 2021 Jun 24;95(14):e0162820. doi: 10.1128/JVI.01628-20. Epub 2021 Jun 24. J Virol. 2021. PMID: 33952641 Free PMC article.
-
An improved animal model for herpesvirus encephalitis in humans.PLoS Pathog. 2020 Mar 30;16(3):e1008445. doi: 10.1371/journal.ppat.1008445. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32226043 Free PMC article.
-
Characterization of a Herpes Simplex Virus 1 (HSV-1) Chimera in Which the Us3 Protein Kinase Gene Is Replaced with the HSV-2 Us3 Gene.J Virol. 2015 Oct 21;90(1):457-73. doi: 10.1128/JVI.02376-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26491159 Free PMC article.
-
Redundant and Specific Roles of A-Type Lamins and Lamin B Receptor in Herpes Simplex Virus 1 Infection.J Virol. 2022 Dec 21;96(24):e0142922. doi: 10.1128/jvi.01429-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448808 Free PMC article.
References
-
- Roizman B, Knipe DM, Whitley RJ (2007) Herpes Simplex Viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA. Fields Virology. 5th ed. Philadelphia, P.A.: Lippincott-Williams & Wilkins.
-
- Hansen TH, Bouvier M (2009) MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol 9: 503-513. doi:10.1038/nri2575. PubMed: 19498380. - DOI - PubMed
-
- Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ (2011) Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol 23: 96-103. doi:10.1016/j.coi.2010.11.005. PubMed: 21146386. - DOI - PubMed
-
- Stevenson PG, May JS, Smith XG, Marques S, Adler H et al. (2002) K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat Immunol 3: 733-740. doi:10.1038/nrg922. PubMed: 12101398. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

