Adhesion to carbon nanotube conductive scaffolds forces action-potential appearance in immature rat spinal neurons
- PMID: 23951361
- PMCID: PMC3741175
- DOI: 10.1371/journal.pone.0073621
Adhesion to carbon nanotube conductive scaffolds forces action-potential appearance in immature rat spinal neurons
Abstract
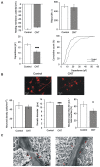
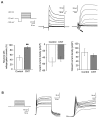
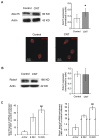
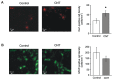
In the last decade, carbon nanotube growth substrates have been used to investigate neurons and neuronal networks formation in vitro when guided by artificial nano-scaled cues. Besides, nanotube-based interfaces are being developed, such as prosthesis for monitoring brain activity. We recently described how carbon nanotube substrates alter the electrophysiological and synaptic responses of hippocampal neurons in culture. This observation highlighted the exceptional ability of this material in interfering with nerve tissue growth. Here we test the hypothesis that carbon nanotube scaffolds promote the development of immature neurons isolated from the neonatal rat spinal cord, and maintained in vitro. To address this issue we performed electrophysiological studies associated to gene expression analysis. Our results indicate that spinal neurons plated on electro-conductive carbon nanotubes show a facilitated development. Spinal neurons anticipate the expression of functional markers of maturation, such as the generation of voltage dependent currents or action potentials. These changes are accompanied by a selective modulation of gene expression, involving neuronal and non-neuronal components. Our microarray experiments suggest that carbon nanotube platforms trigger reparative activities involving microglia, in the absence of reactive gliosis. Hence, future tissue scaffolds blended with conductive nanotubes may be exploited to promote cell differentiation and reparative pathways in neural regeneration strategies.
Conflict of interest statement
Figures

References
-
- Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8: 457-470. doi:10.1038/nmat2441. PubMed: 19458646. - DOI - PubMed
-
- Dvir T, Timko BP, Kohane DS, Langer R (2011) Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6: 13-22. doi:10.1038/nnano.2010.246. PubMed: 21151110. - DOI - PMC - PubMed
-
- Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10: 21-33. doi:10.1038/nrm2593. PubMed: 19197329. - DOI - PubMed
-
- Brunetti V, Maiorano G, Rizzello L, Sorce B, Sabella S et al. (2010) Neurons sense nanoscale roughness with nanometer sensitivity. Proc Natl Acad Sci U S A 107: 6264-6269. doi:10.1073/pnas.0914456107. PubMed: 20308580. - DOI - PMC - PubMed
-
- Atala A (2009) Engineering organs. Curr Opin Biotechnol 20: 575-592. doi:10.1016/j.copbio.2009.10.003. PubMed: 19896823. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

