The mTOR pathway and integrating immune regulation
- PMID: 23952610
- PMCID: PMC3839643
- DOI: 10.1111/imm.12162
The mTOR pathway and integrating immune regulation
Abstract
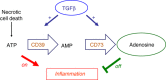
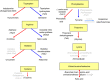
The mammalian target of rapamycin (mTOR) pathway is an important integrator of nutrient-sensing signals in all mammalian cells, and acts to coordinate the cell proliferation with the availability of nutrients such as glucose, amino acids and energy (oxygen and ATP). A large part of the immune response depends on the proliferation and clonal expansion of antigen-specific T cells, which depends on mTOR activation, and the pharmacological inhibition of this pathway by rapamycin is therefore potently immunosuppressive. It is only recently, however, that we have started to understand the more subtle details of how the mTOR pathway is involved in controlling the differentiation of effector versus memory CD8(+) T cells and the decision to generate different CD4(+) helper T-cell subsets. In particular, this review will focus on how nutrient sensing via mTOR controls the expression of the master transcription factor for regulatory T cells in order to maintain the balance between tolerance and inflammation.
Keywords: FOXP3; metabolism; nutrient sensing; regulatory T cells.
© 2013 John Wiley & Sons Ltd.
Figures


References
-
- Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22:655–61. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. - PubMed
-
- Buttgereit F, Brand MD, Muller M. ConA induced changes in energy metabolism of rat thymocytes. Biosci Rep. 1992;12:381–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

