Continuing efficacy of milnacipran following long-term treatment in fibromyalgia: a randomized trial
- PMID: 23953493
- PMCID: PMC3978750
- DOI: 10.1186/ar4268
Continuing efficacy of milnacipran following long-term treatment in fibromyalgia: a randomized trial
Abstract
Introduction: Previous studies of long-term treatment response in fibromyalgia and other chronic pain states have generally been limited to approximately one year, leaving questions about the longer-term durability of response. The purpose of this study was to demonstrate continuing efficacy of milnacipran by characterizing changes in pain and other fibromyalgia symptoms after discontinuing long-term treatment. The mean length of milnacipran treatment at the time of randomized withdrawal was 36.1 months from initial exposure to milnacipran (range, 17.9 to 54.4 months).
Methods: After completing a long-term, open-label, lead-in study of milnacipran (which followed varying periods of exposure in previous studies), adult patients with fibromyalgia entered the four-week open-label period of the current study for evaluation of ongoing treatment response. After the four-week period to confirm new baseline status, 151 patients taking milnacipran ≥100 mg/day and reporting ≥50% improvement from pre-milnacipran exposure in Visual Analogue Scale (VAS) pain scores were classified as responders. These responders entered the 12-week, double-blind withdrawal period in which they were randomized 2:1 to continue milnacipran or switched to placebo. The prespecified primary parameter was loss of therapeutic response (LTR), defined as increase in VAS pain score to <30% reduction from pre-milnacipran exposure or worsening of fibromyalgia requiring alternative treatment. Adverse events and vital signs were also monitored.
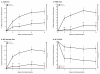
Results: Time to LTR was shorter in patients randomized to placebo than in patients continuing milnacipran (P < 0.001). Median time to LTR was 56 days with placebo and was not calculable for milnacipran, because less than half of the latter group of patients lost therapeutic response by study end. Additionally, 81% of patients continuing on milnacipran maintained clinically meaningful pain response (≥30% improvement from pre-milnacipran exposure), compared with 58% of patients switched to placebo (sensitivity analysis II; P < 0.001). The incidences of treatment-emergent adverse events were 58% and 47% for placebo and milnacipran, respectively. Mean decreases in blood pressure and heart rate were found in both groups, with greater decreases for patients switched to placebo.
Conclusions: Continuing efficacy of milnacipran was demonstrated by the loss of effect following withdrawal of treatment in patients who received an average of three years of milnacipran treatment.
Trial registration: ClinicalTrials.gov: NCT01014585.
Figures


References
-
- MacFarlane GJ, Thomas E, Papageorgiou AC, Schollum J, Croft PR, Silman AJ. The natural history of chronic pain in the community: a better prognosis than in the clinic? J Rheumatol. 1996;15:1617–1620. - PubMed
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;15:160–172. doi: 10.1002/art.1780330203. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

