Glucocorticoid receptor signaling in health and disease
- PMID: 23953592
- PMCID: PMC3951203
- DOI: 10.1016/j.tips.2013.07.003
Glucocorticoid receptor signaling in health and disease
Abstract
Glucocorticoids are steroid hormones regulated in a circadian and stress-associated manner to maintain various metabolic and homeostatic functions that are necessary for life. Synthetic glucocorticoids are widely prescribed drugs for many conditions including asthma, chronic obstructive pulmonary disease (COPD), and inflammatory disorders of the eye. Research in the past few years has begun to unravel the profound complexity of glucocorticoid signaling and has contributed remarkably to improved therapeutic strategies. Glucocorticoids signal through the glucocorticoid receptor (GR), a member of the superfamily of nuclear receptors, in both genomic and non-genomic ways in almost every tissue in the human body. In this review, we provide an update on glucocorticoid receptor signaling and highlight the role of GR signaling in physiological and pathophysiological conditions in the major organ systems in the human body.
Keywords: glucocorticoid-response element; inflammation; nuclear receptor; stress hormones.
Published by Elsevier Ltd.
Figures
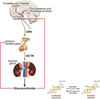
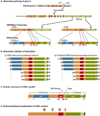

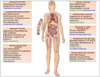
References
-
- Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54–67. - PubMed
-
- Buttgereit F. A fresh look at glucocorticoids how to use an old ally more effectively. Bull NYU Hosp Jt Dis. 2012;70(Suppl 1):26–29. - PubMed
-
- Baschant U, et al. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:645–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

