Gut sensing of potassium intake and its role in potassium homeostasis
- PMID: 23953802
- PMCID: PMC3748407
- DOI: 10.1016/j.semnephrol.2013.04.005
Gut sensing of potassium intake and its role in potassium homeostasis
Abstract
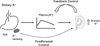
Extracellular K(+) homeostasis has been explained by feedback mechanisms in which changes in extracellular K(+) concentration drive renal K(+) excretion directly or indirectly via stimulating aldosterone secretion. However, this cannot explain meal-induced kaliuresis, which often occurs without increases in plasma K(+) or aldosterone concentrations. Recent studies have produced evidence supporting a feedforward control in which gut sensing of dietary K(+) increases renal K(+) excretion (and extrarenal K(+) uptake) independent of plasma K(+) concentrations, namely, a gut factor. This review focuses on these new findings and discusses the role of gut factor in acute and chronic regulation of extracellular K(+) as well as in the beneficial effects of high K(+) intake on the cardiovascular system.
Keywords: Potassium excretion; feedback control; feedforward control; potassium adaptation; potassium balance.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


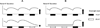
Similar articles
-
Recent advances in understanding integrative control of potassium homeostasis.Annu Rev Physiol. 2009;71:381-401. doi: 10.1146/annurev.physiol.010908.163241. Annu Rev Physiol. 2009. PMID: 18759636 Free PMC article. Review.
-
Gut sensing of dietary K⁺ intake increases renal K⁺excretion.Am J Physiol Regul Integr Comp Physiol. 2011 Aug;301(2):R421-9. doi: 10.1152/ajpregu.00095.2011. Epub 2011 May 4. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21543632 Free PMC article.
-
A mathematical model of potassium homeostasis: Effect of feedforward and feedback controls.PLoS Comput Biol. 2022 Dec 20;18(12):e1010607. doi: 10.1371/journal.pcbi.1010607. eCollection 2022 Dec. PLoS Comput Biol. 2022. PMID: 36538563 Free PMC article.
-
Role of pituitary in K+ homeostasis: impaired renal responses to altered K+ intake in hypophysectomized rats.Am J Physiol Regul Integr Comp Physiol. 2013 Jun 15;304(12):R1166-74. doi: 10.1152/ajpregu.00495.2012. Epub 2013 Apr 17. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23594607 Free PMC article.
-
Aldosterone and potassium homeostasis.Kidney Int. 1996 Jun;49(6):1738-42. doi: 10.1038/ki.1996.258. Kidney Int. 1996. PMID: 8743488 Review.
Cited by
-
Hydroelectrolytic Disorder in COVID-19 patients: Evidence Supporting the Involvement of Subfornical Organ and Paraventricular Nucleus of the Hypothalamus.Neurosci Biobehav Rev. 2021 May;124:216-223. doi: 10.1016/j.neubiorev.2021.02.008. Epub 2021 Feb 10. Neurosci Biobehav Rev. 2021. PMID: 33577841 Free PMC article. Review.
-
Hyperkalemia in Chronic Kidney Disease: Links, Risks and Management.Int J Nephrol Renovasc Dis. 2022 Aug 2;15:215-228. doi: 10.2147/IJNRD.S326464. eCollection 2022. Int J Nephrol Renovasc Dis. 2022. PMID: 35942480 Free PMC article. Review.
-
Estimating in vivo potassium distribution and fluxes with stable potassium isotopes.Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C410-C420. doi: 10.1152/ajpcell.00351.2021. Epub 2022 Jan 26. Am J Physiol Cell Physiol. 2022. PMID: 35080924 Free PMC article.
-
Regulation of muscle potassium: exercise performance, fatigue and health implications.Eur J Appl Physiol. 2021 Mar;121(3):721-748. doi: 10.1007/s00421-020-04546-8. Epub 2021 Jan 4. Eur J Appl Physiol. 2021. PMID: 33392745 Review.
-
Directing two-way traffic in the kidney: A tale of two ions.J Gen Physiol. 2022 Oct 3;154(10):e202213179. doi: 10.1085/jgp.202213179. Epub 2022 Sep 1. J Gen Physiol. 2022. PMID: 36048011 Free PMC article.
References
-
- Stokes JB. Potassium intoxication: pathogenesis and treatment. In: Seldin DW, Giebisch G, editors. The regulation of potassium balance. New York: Raven; 1989.
-
- McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–F974. - PubMed
-
- Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

