β2-Adrenoceptor agonists in the regulation of mitochondrial biogenesis
- PMID: 23954364
- PMCID: PMC3987705
- DOI: 10.1016/j.bmcl.2013.07.052
β2-Adrenoceptor agonists in the regulation of mitochondrial biogenesis
Abstract
The stimulation of mitochondrial biogenesis (MB) via cell surface G-protein coupled receptors is a promising strategy for cell repair and regeneration. Here we report the specificity and chemical rationale of a panel of β2-adrenoceptor agonists with regards to MB. Using primary cultures of renal cells, a diverse panel of β2-adrenoceptor agonists elicited three distinct phenotypes: full MB, partial MB, and non-MB. Full MB compounds had efficacy in the low nanomolar range and represent two chemical scaffolds containing three distinct chemical clusters. Interestingly, the MB phenotype did not correlate with reported receptor affinity or chemical similarity. Chemical clusters were then subjected to pharmacophore modeling creating two models with unique and distinct features, consisting of five conserved amongst full MB compounds were identified. The two discrete pharmacophore models were coalesced into a consensus pharmacophore with four unique features elucidating the spatial and chemical characteristics required to stimulate MB.
Keywords: Adrenoceptor; Biogenesis; Chemical similarity; Clustering; Mitochondria; Pharmacophore; Renal.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
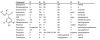



References
-
- Hirsch T, Susin SA, Marzo I, Marchetti P, Zamzami N, Kroemer G. Cell Biol and Tox. 1998;14:141–145. - PubMed
-
- Birch-Machin MA, Turnbull DM. In: In Methods in Cell Biology. Pon Liza A, EAS, editors. Academic Press; 2001. pp. 97–117. - PubMed
-
- Wallace KB, Starkov AA. Ann Rev Pharm and Tox. 2000;40:353–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM084147/GM/NIGMS NIH HHS/United States
- R43 GM097770/GM/NIGMS NIH HHS/United States
- R01 DK071997/DK/NIDDK NIH HHS/United States
- DK071997/DK/NIDDK NIH HHS/United States
- T32 GM08716/GM/NIGMS NIH HHS/United States
- F32 ES020103/ES/NIEHS NIH HHS/United States
- GM084147/GM/NIGMS NIH HHS/United States
- F32 ES020103-01/ES/NIEHS NIH HHS/United States
- T32 ES012878/ES/NIEHS NIH HHS/United States
- R43 ES019378/ES/NIEHS NIH HHS/United States
- T32 GM008716/GM/NIGMS NIH HHS/United States
- S10 RR027122/RR/NCRR NIH HHS/United States
- I01 BX000851/BX/BLRD VA/United States
- R44 ES019378/ES/NIEHS NIH HHS/United States
- T32 CA119945-04/CA/NCI NIH HHS/United States
- ES012878/ES/NIEHS NIH HHS/United States
- T32 CA119945/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

