SIV antigen-specific effects on immune responses induced by vaccination with DNA electroporation and plasmid IL-12
- PMID: 23954384
- PMCID: PMC7570371
- DOI: 10.1016/j.vaccine.2013.08.011
SIV antigen-specific effects on immune responses induced by vaccination with DNA electroporation and plasmid IL-12
Abstract
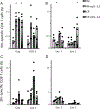
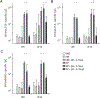
Molecular adjuvants are important for augmenting or modulating immune responses induced by DNA vaccination. Promising results have been obtained using IL-12 expression plasmids in a variety of disease models including the SIV model of HIV infection. We used a mouse model to evaluate plasmid IL-12 (pIL-12) in a DNA prime, recombinant adenovirus serotype 5 (rAd5) boost regimen specifically to evaluate the effect of IL-12 expression on cellular and humoral immunity induced against both SIVmac239 Gag and Env antigens. Priming with electroporated (EP) DNA+pIL-12 resulted in a 2-4-fold enhanced frequency of Gag-specific CD4 T cells which was maintained through the end of the study irrespective of the pIL-12 dose, while memory Env-specific CD4+T cells were maintained only at the low dose of pIL-12. There was little positive effect of pIL-12 on the humoral response to Env, and in fact, high dose pIL-12 dramatically reduced SIV Env-specific IgG. Additionally, both doses of pIL-12 diminished the frequency of CD8 T-cells after DNA prime, although a rAd5 boost recovered CD8 responses regardless of the pIL-12 dose. In this prime-boost regimen, we have shown that a high dose pIL-12 can systemically reduce Env-specific humoral responses and CD4T cell frequency, but not Gag-specific CD4+ T cells. These data indicate that it is important to independently characterize individual SIV or HIV antigen immunogenicity in multi-antigenic vaccines as a function of adjuvant dose.
Keywords: Adenovirus; Antibody; DNA; IL-12; T cell.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
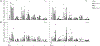



References
-
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. Journal of Infectious Diseases 1998;178(July (1)):92–100. - PubMed
-
- Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA. Delivery of DNA vaccines using electroporation. Methods in Molecular Medicine 2006;127:73–82. - PubMed
-
- Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000;290(October (5491)):486–92. - PubMed
-
- Buchan S, Gronevik E, Mathiesen I, King CA, Stevenson FK, Rice J. Electroporation as a prime/boost strategy for naked DNA vaccination against a tumor antigen. Journal of Immunology 2005;174(May (10)):6292–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

