High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression
- PMID: 23954404
- PMCID: PMC3838664
- DOI: 10.1016/j.reprotox.2013.07.026
High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression
Abstract
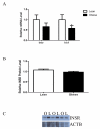
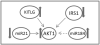
Insulin regulates ovarian phosphatidylinositol-3-kinase (PI3 K) signaling, important for primordial follicle viability and growth activation. This study investigated diet-induced obesity impacts on: (1) insulin receptor (Insr) and insulin receptor substrate 1 (Irs1); (2) PI3K components (Kit ligand (Kitlg), kit (c-Kit), protein kinase B alpha (Akt1) and forkhead transcription factor subfamily 3 (Foxo3a)); (3) xenobiotic biotransformation (microsomal epoxide hydrolase (Ephx1), Cytochrome P450 isoform 2E1 (Cyp2e1), Glutathione S-transferase (Gst) isoforms mu (Gstm) and pi (Gstp)) and (4) microRNA's 184, 205, 103 and 21 gene expression. INSR, GSTM and GSTP protein levels were also measured. Obese mouse ovaries had decreased Irs1, Foxo3a, Cyp2e1, MiR-103, and MiR-21 but increased Kitlg, Akt1, and miR-184 levels relative to lean littermates. These results support that diet-induced obesity potentially impairs ovarian function through aberrant gene expression.
Keywords: Obesity; Ovary; Phosphatidylinositol-3 kinase.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

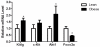



Similar articles
-
Progressive obesity alters ovarian insulin, phosphatidylinositol-3 kinase, and chemical metabolism signaling pathways and potentiates ovotoxicity induced by phosphoramide mustard in mice.Biol Reprod. 2017 Feb 1;96(2):478-490. doi: 10.1095/biolreprod.116.143818. Biol Reprod. 2017. PMID: 28203716
-
Impact of obesity on ovotoxicity induced by 7,12-dimethylbenz[a]anthracene in mice.Biol Reprod. 2014 Mar 27;90(3):68. doi: 10.1095/biolreprod.113.114215. Print 2014 Mar. Biol Reprod. 2014. PMID: 24501177 Free PMC article.
-
Heat Stress Alters Ovarian Insulin-Mediated Phosphatidylinositol-3 Kinase and Steroidogenic Signaling in Gilt Ovaries.Biol Reprod. 2015 Jun;92(6):148. doi: 10.1095/biolreprod.114.126714. Epub 2015 Apr 29. Biol Reprod. 2015. PMID: 25926439
-
Protective role for ovarian glutathione S-transferase isoform pi during 7,12-dimethylbenz[a]anthracene-induced ovotoxicity.Toxicol Appl Pharmacol. 2012 Apr 15;260(2):201-8. doi: 10.1016/j.taap.2012.02.014. Epub 2012 Mar 1. Toxicol Appl Pharmacol. 2012. PMID: 22406437 Free PMC article.
-
The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression.Pharmacol Ther. 2007 Jan;113(1):88-120. doi: 10.1016/j.pharmthera.2006.07.004. Epub 2006 Nov 13. Pharmacol Ther. 2007. PMID: 17097148 Free PMC article. Review.
Cited by
-
Intergenerational Hyperglycemia Impairs Mitochondrial Function and Follicular Development and Causes Oxidative Stress in Rat Ovaries Independent of the Consumption of a High-Fat Diet.Nutrients. 2023 Oct 17;15(20):4407. doi: 10.3390/nu15204407. Nutrients. 2023. PMID: 37892483 Free PMC article.
-
miR-21, miR-221, miR-29 and miR-34 are distinguishable molecular features of a metabolically unhealthy phenotype in young adults.PLoS One. 2024 Apr 25;19(4):e0300420. doi: 10.1371/journal.pone.0300420. eCollection 2024. PLoS One. 2024. PMID: 38662716 Free PMC article.
-
Red pitaya juice supplementation ameliorates energy balance homeostasis by modulating obesity-related genes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats.BMC Complement Altern Med. 2016 Jul 26;16:243. doi: 10.1186/s12906-016-1200-3. BMC Complement Altern Med. 2016. PMID: 27456968 Free PMC article.
-
The Role of Bioactive Dietary Components in Modulating miRNA Expression in Colorectal Cancer.Nutrients. 2016 Sep 26;8(10):590. doi: 10.3390/nu8100590. Nutrients. 2016. PMID: 27681738 Free PMC article. Review.
-
PFOA-Induced Ovotoxicity Differs Between Lean and Obese Mice With Impacts on Ovarian Reproductive and DNA Damage Sensing and Repair Proteins.Toxicol Sci. 2022 Nov 23;190(2):173-188. doi: 10.1093/toxsci/kfac104. Toxicol Sci. 2022. PMID: 36214631 Free PMC article.
References
-
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:w822–831. - PubMed
-
- Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update. 2007;13:433–444. - PubMed
-
- Devi S. Progress on childhood obesity patchy in the USA. Lancet. 2008;371:105–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

