More surprises lying ahead. The endocannabinoids keep us guessing
- PMID: 23954677
- PMCID: PMC3855347
- DOI: 10.1016/j.neuropharm.2013.07.026
More surprises lying ahead. The endocannabinoids keep us guessing
Abstract
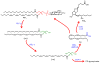
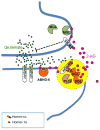
The objective of this review is to point out some important facts that we don't know about endogenous cannabinoids - lipid-derived signaling molecules that activate CB1 cannabinoid receptors and play key roles in motivation, emotion and energy balance. The first endocannabinoid substance to be discovered, anandamide, was isolated from brain tissue in 1992. Research has shown that this molecule is a bona fide brain neurotransmitter involved in the regulation of stress responses and pain, but the molecular mechanisms that govern its formation and the neural pathways in which it is employed are still unknown. There is a general consensus that enzyme-mediated cleavage, catalyzed by fatty acid amide hydrolase (FAAH), terminates the biological actions of anandamide, but there are many reasons to believe that other as-yet-unidentified proteins are also involved in this process. We have made significant headway in understanding the second arrived in the endocannabinoid family, 2-arachidonoyl-sn-glycerol (2-AG), which was discovered three years after anandamide. Researchers have established some of the key molecular players involved in 2-AG formation and deactivation, localized them to specific synaptic components, and showed that their assembly into a multi-molecular protein complex (termed the '2-AG signalosome') allows 2-AG to act as a retrograde messenger at excitatory synapses of the brain. Basic questions that remain to be answered pertain to the exact molecular composition of the 2-AG signalosome, its regulation by neural activity and its potential role in the actions of drugs of abuse such as Δ(9)-THC and cocaine. This article is part of a Special Issue entitled 'NIDA 40th Anniversary Issue'.
Keywords: Anandamide; Cannabinoid receptor; Cocaine; Drugs of abuse; Endocannabinoid.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Allen AC, Gammon CM, Ousley AH, McCarthy KD, Morell P. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. 1992;58:1130–1139. - PubMed
-
- Barinaga M. Pot, heroin unlock new areas for neuroscience. Science. 1992;258:1882–1884. - PubMed
-
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

