Dopamine D₂ and acetylcholine α7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area
- PMID: 23954803
- PMCID: PMC4056450
- DOI: 10.1016/j.neuroscience.2013.08.008
Dopamine D₂ and acetylcholine α7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area
Abstract
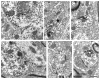
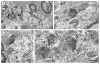
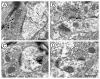
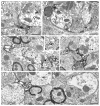
Alpha7 nicotinic acetylcholine receptors (α7nAChRs) mediate nicotine-induced burst-firing of dopamine neurons in the ventral tegmental area (VTA), a limbic brain region critically involved in reward and in dopamine D2 receptor (D2R)-related cortical dysfunctions associated with psychosis. The known presence of α7nAChRs and Gi-coupled D2Rs in dopamine neurons of the VTA suggests that these receptors are targeted to at least some of the same neurons in this brain region. To test this hypothesis, we used electron microscopic immunolabeling of antisera against peptide sequences of α7nACh and D2 receptors in the mouse VTA. Dual D2R and α7nAChR labeling was seen in many of the same somata (co-localization over 97%) and dendrites (co-localization over 49%), where immunoreactivity for each of the receptors was localized to endomembranes as well as to non-synaptic or synaptic plasma membranes often near excitatory-type synapses. In comparison with somata and dendrites, many more small axons and axon terminals were separately labeled for each of the receptors. Thus, single-labeled axon terminals were predominant for both α7nAChR (57.9%) and D2R (89.0%). The majority of the immunolabeled axonal profiles contained D2R-immunoreactivity (81.6%) and formed either symmetric or asymmetric synapses consistent with involvement in the release of both inhibitory and excitatory transmitters. Of 160 D2R-labeled terminals, 81.2% were presynaptic to dendrites that expressed α7nAChR alone or together with the D2R. Numerous glial processes inclusive of those enveloping either excitatory- or inhibitory-type synapses also contained single labeling for D2R (n=152) and α7nAChR (n=561). These results suggest that classic antipsychotic drugs, all of which block the D2R, may facilitate α7nAChR-mediated burst-firing by elimination of D2R-dependent inhibition in neurons expressing both receptors as well as by indirect pre-synaptic and glial mechanisms.
Keywords: ABC; ACh; Avidin–biotin complex; BSA; D(2)R; G-protein-coupled inwardly rectifying potassium; GIRK; MLA; N-methyl-d-aspartate receptor; NAc; NMDA; PB; PBS; TS; Tris-buffered saline; VTA; acetylcholine; addiction; alpha7 nicotinic acetylcholine receptors; bovine serum albumin; dopamine D(2) receptor; electron microscopic immunolabeling; mesocorticolimbic; methyllycaconitine; nAChR; nicotinic acetylcholine receptor; nucleus accumbens; phosphate buffer; phosphate-buffered saline; reward; schizophrenia; ventral tegmental area; α7nAChRs.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Acetylcholine α7 nicotinic and dopamine D2 receptors are targeted to many of the same postsynaptic dendrites and astrocytes in the rodent prefrontal cortex.Synapse. 2011 Dec;65(12):1350-67. doi: 10.1002/syn.20977. Synapse. 2011. PMID: 21858872 Free PMC article.
-
Region-specific targeting of dopamine D2-receptors and somatodendritic vesicular monoamine transporter 2 (VMAT2) within ventral tegmental area subdivisions.Synapse. 2002 Aug;45(2):113-24. doi: 10.1002/syn.10092. Synapse. 2002. PMID: 12112404
-
Spatial and intracellular relationships between the alpha7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse.Neuroscience. 2009 Jul 21;161(4):1091-103. doi: 10.1016/j.neuroscience.2009.04.024. Epub 2009 Apr 15. Neuroscience. 2009. PMID: 19374941 Free PMC article.
-
Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter.J Comp Neurol. 1999 Jul 26;410(2):197-210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. J Comp Neurol. 1999. PMID: 10414527 Review.
-
Electron microscopic immunolabeling of transporters and receptors identifies transmitter-specific functional sites envisioned in Cajal's neuron.Prog Brain Res. 2002;136:145-55. doi: 10.1016/s0079-6123(02)36014-x. Prog Brain Res. 2002. PMID: 12143378 Review.
Cited by
-
Dopamine D2 autoreceptor interactome: Targeting the receptor complex as a strategy for treatment of substance use disorder.Pharmacol Ther. 2020 Sep;213:107583. doi: 10.1016/j.pharmthera.2020.107583. Epub 2020 May 27. Pharmacol Ther. 2020. PMID: 32473160 Free PMC article. Review.
-
Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex.J Neurosci. 2014 Mar 5;34(10):3706-18. doi: 10.1523/JNEUROSCI.0623-13.2014. J Neurosci. 2014. PMID: 24599469 Free PMC article.
-
Crucial Role of Dopamine D2 Receptor Signaling in Nicotine-Induced Conditioned Place Preference.Mol Neurobiol. 2019 Dec;56(12):7911-7928. doi: 10.1007/s12035-019-1635-x. Epub 2019 May 25. Mol Neurobiol. 2019. PMID: 31129809
-
Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity.Neuropharmacology. 2016 Jun;105:618-629. doi: 10.1016/j.neuropharm.2016.02.028. Epub 2016 Feb 24. Neuropharmacology. 2016. PMID: 26921769 Free PMC article.
-
Negative and positive allosteric modulators of the α7 nicotinic acetylcholine receptor regulates the ability of adolescent binge alcohol exposure to enhance adult alcohol consumption.Front Behav Neurosci. 2023 Apr 4;16:954319. doi: 10.3389/fnbeh.2022.954319. eCollection 2022. Front Behav Neurosci. 2023. PMID: 37082421 Free PMC article.
References
-
- Araque A, Parpura V, Sanzgiri R, Haydon P. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
-
- Arnaiz-Cot J, Gonzalez J, Sobrado M, Baldelli P, Carbone E, Gandia L, Garcia A, Hernandez-Guijo J. Allosteric modulation of alpha 7 nicotinic receptors selectively depolarizes hippocampal interneurons, enhancing spontaneous GABAergic transmission. Eur J Neurosci. 2008;27:1097–1110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

