Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond
- PMID: 23955016
- PMCID: PMC3825489
- DOI: 10.1007/s00109-013-1074-5
Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond
Abstract
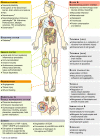
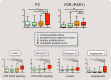
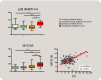
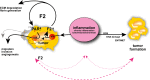
Thrombin is the protease involved in blood coagulation. Its deregulation can lead to hemostatic abnormalities, which range from subtle subclinical to serious life-threatening coagulopathies, i.e., during septicemia. Additionally, thrombin plays important roles in many (patho)physiological conditions that reach far beyond its well-established role in stemming blood loss and thrombosis, including embryonic development and angiogenesis but also extending to inflammatory processes, complement activation, and even tumor biology. In this review, we will address thrombin's broad roles in diverse (patho)physiological processes in an integrative way. We will also discuss thrombin as an emerging major target for novel therapies.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

