The trifunctional antibody catumaxomab amplifies and shapes tumor-specific immunity when applied to gastric cancer patients in the adjuvant setting
- PMID: 23955093
- PMCID: PMC4162043
- DOI: 10.4161/hv.26065
The trifunctional antibody catumaxomab amplifies and shapes tumor-specific immunity when applied to gastric cancer patients in the adjuvant setting
Abstract
Background: Patients with gastric cancer benefit from perioperative chemotherapy, however, treatment is toxic and many patients will relapse. The trifunctional antibody catumaxomab targets EpCAM on tumor cells, CD3 on T cells, and the Fcγ-receptor of antigen-presenting cells. While in Europe catumaxomab is approved for treating malignant ascites, it has not been investigated in the perioperative setting and its exact immunological mode of action is unclear.
Methods: In our study, gastric cancer patients received neoadjuvant platinum-based chemotherapy, one intraoperative application of catumaxomab, and 4 postoperative doses of intraperitoneal catumaxomab. Immunomonitoring was performed in 6 patients before surgery, after completion of catumaxomab treatment, and one month later.
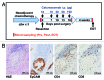
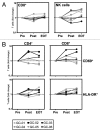
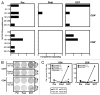
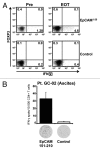
Results: Intraperitoneal application of catumaxomab caused an increased expression of activation markers on the patients' T cells. This was accompanied by a transient decrease in numbers of CXCR3(+) effector T cells with a T-helper (Th)-1 phenotype in the peripheral blood. All patients evidenced pre-existing EpCAM-specific CD4(+) and/or CD8(+) T cells. While these cells transiently disappeared from the blood stream after intraperitoneal application of catumaxomab, we detected increased numbers of peripheral EpCAM-specific cells and a modified EpCAM-specific T-cell repertoire 4 weeks after completion of treatment. Finally, catumaxomab also amplified humoral immunity to tumor antigens other than EpCAM.
Conclusions: Our findings suggest that catumaxomab exerts its clinical effects by (1) activating peripheral T cells, (2) redistributing effector T cells from the blood into peripheral tissues, (3) expanding and shaping of the pre-existing EpCAM-specific T-cell repertoire, and (4) spreading of anti-tumor immunity to different tumor antigens.
Keywords: EpCAM; T cells; adjuvant immunotherapy; catumaxomab; gastric cancer; tumor immunology.
Figures


Similar articles
-
Treatment of gastric peritoneal carcinomatosis by combining complete surgical resection of lesions and intraperitoneal immunotherapy using catumaxomab.BMC Cancer. 2014 Mar 4;14:148. doi: 10.1186/1471-2407-14-148. BMC Cancer. 2014. PMID: 24589307 Free PMC article. Clinical Trial.
-
Immunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3).Cancer Res. 2012 Jan 1;72(1):24-32. doi: 10.1158/0008-5472.CAN-11-2235. Epub 2011 Nov 1. Cancer Res. 2012. PMID: 22044753 Clinical Trial.
-
Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM×anti-CD3).Gynecol Oncol. 2015 Aug;138(2):343-51. doi: 10.1016/j.ygyno.2015.06.003. Epub 2015 Jun 3. Gynecol Oncol. 2015. PMID: 26049121 Clinical Trial.
-
Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy.Cancer Treat Rev. 2010 Oct;36(6):458-67. doi: 10.1016/j.ctrv.2010.03.001. Epub 2010 Mar 27. Cancer Treat Rev. 2010. PMID: 20347527 Review.
-
Catumaxomab: a bispecific trifunctional antibody.Drugs Today (Barc). 2009 Aug;45(8):589-97. doi: 10.1358/dot.2009.45.8.1401103. Drugs Today (Barc). 2009. PMID: 19927225 Review.
Cited by
-
Dawn of Monitoring Regulatory T Cells in (Pre-)clinical Studies: Their Relevance Is Slowly Recognised.Front Med (Lausanne). 2020 Apr 2;7:91. doi: 10.3389/fmed.2020.00091. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32300597 Free PMC article.
-
CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease.Int J Mol Sci. 2023 Jan 31;24(3):2696. doi: 10.3390/ijms24032696. Int J Mol Sci. 2023. PMID: 36769019 Free PMC article. Review.
-
Cancer vaccines compensate for the insufficient induction of protective tumor-specific immunity of CD3 bispecific antibody therapy.J Immunother Cancer. 2025 Jan 11;13(1):e010331. doi: 10.1136/jitc-2024-010331. J Immunother Cancer. 2025. PMID: 39800374 Free PMC article.
-
T Cell Bispecific Antibodies: An Antibody-Based Delivery System for Inducing Antitumor Immunity.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1172. doi: 10.3390/ph14111172. Pharmaceuticals (Basel). 2021. PMID: 34832954 Free PMC article. Review.
-
T Cell-Redirecting Bispecific Antibodies in Multiple Myeloma: Optimal Dosing Schedule and Duration of Treatment.Blood Cancer Discov. 2024 Nov 1;5(6):388-399. doi: 10.1158/2643-3230.BCD-24-0124. Blood Cancer Discov. 2024. PMID: 39321136 Free PMC article. Review.
References
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
