Utility of physiologically based modeling and preclinical in vitro/in vivo data to mitigate positive food effect in a BCS class 2 compound
- PMID: 23955148
- PMCID: PMC3755159
- DOI: 10.1208/s12249-013-0018-2
Utility of physiologically based modeling and preclinical in vitro/in vivo data to mitigate positive food effect in a BCS class 2 compound
Abstract
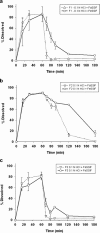
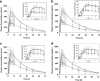
Physiologically based pharmacokinetic (PBPK) modeling has become a useful tool to estimate the performance of orally administrated drugs. Here, we described multiple in silico/in vitro/in vivo tools to support formulation development toward mitigating the positive food effect of NVS123, a weak base with a pH-dependent and limited solubility. Administered orally with high-fat meal, NVS123 formulated as dry filled capsules displayed a positive food effects in humans. Three alternative formulations were developed and assessed in in vitro and in vivo preclinical and/or clinical studies. By integrating preclinical in vitro and in vivo data, the PBPK model successfully estimated the magnitude of food effects and the predicted values were within ± 30% of the observed results. A model-guided parameter sensitivity analysis illustrated that enhanced solubility and longer precipitation times under fed condition were the main reason for enhanced NVS123's exposure in presence of food. Eventually, exposure after an amorphous formulation was found to be not significantly altered because of remarkably enhanced intestinal solubility and reduced precipitation. Gastroplus population simulations also suggested that the amorphous formulation is promising in mitigating a clinically significant food effect. Overall, these efforts supported the rationale of clinical investigation of the new formulation, and more importantly, highlighted a practical application of PBPK modeling solving issues of undesirable food effects in weakly basic compounds based on preclinical in vitro/in vivo data.
Figures




References
-
- Benet LZ. WCY. Using a biopharmaceutics drug disposition classification system to predict bioavailability and elimination characteristics of new molecular entities. Somerset, NJ: NJDMDG; 2006.
-
- FDA. Food-Effect Bioavailability and Fed Bioequivalence Studies. Guidance for Industry 2002. Available from: http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf. Accessed 02 Jun 2012
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

