Substandard and counterfeit medicines: a systematic review of the literature
- PMID: 23955188
- PMCID: PMC3752049
- DOI: 10.1136/bmjopen-2013-002923
Substandard and counterfeit medicines: a systematic review of the literature
Abstract
Objective: To explore the evidence available of poor-quality (counterfeit and substandard) medicines in the literature.
Design: Systematic review.
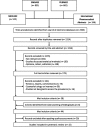
Data sources: Databases used were EMBASE, MEDLINE, PubMed and the International Pharmaceutical Abstracts, including articles published till January 2013.
Eligibility criteria: Prevalence studies containing original data. WHO definitions (1992) used for counterfeit and substandard medicines.
Study appraisal and synthesis: Two reviewers independently scored study methodology against recommendations from the MEDQUARG Checklist. Studies were classified according to the World Bank classification of countries by income.
Data extraction: Data extracted: place of study; type of drugs sampled; sample size; percentage of substandard/counterfeit medicines; formulations included; origin of the drugs; chemical analysis and stated issues of counterfeit/substandard medicines.
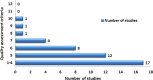
Results: 44 prevalence studies were identified, 15 had good methodological quality. They were conducted in 25 different countries; the majority were in low-income countries (11) and/or lower middle-income countries (10). The median prevalence of substandard/counterfeit medicines was 28.5% (range 11-48%). Only two studies differentiated between substandard and counterfeit medicines. Prevalence data were limited to antimicrobial drugs (all 15 studies). 13 studies involved antimalarials, 6 antibiotics and 2 other medications. The majority of studies (93%) contained samples with inadequate amounts of active ingredients. The prevalence of substandard/counterfeit antimicrobials was significantly higher when purchased from unlicensed outlets (p<0.000; 95% CI 0.21 to 0.32). No individual data about the prevalence in upper middle-income countries and high-income countries were available.
Limitations: Studies with strong methodology were few. The majority did not differentiate between substandard and counterfeit medicines. Most studies assessed only a single therapeutic class of antimicrobials.
Conclusions: The prevalence of poor-quality antimicrobial medicines is widespread throughout Africa and Asia in lower income countries and lower middle-income countries . The main problem identified was inadequate amounts of the active ingredients.
Keywords: anti-infective; counterfeit drug; drug counterfeiting; fake drug; substandard drug.
Figures
References
-
- Caudron JM, Ford N, Henkens M, et al. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health 2008;13:1062–72 - PubMed
-
- Mackey TK, Liang BA. The global counterfeit drug trade: patient safety and public health risks. J Pharm Sci 2011;100:4571–9 - PubMed
-
- Liang B. Fade to black: importation and counterfeit drugs. Am J Law Med 2006;32:279–323 - PubMed
-
- European Commission Report on EU customs enforcement of intellectual property rights: results at the EU border. 2011. http://ec.europa.eu/taxation_customs/resources/documents/customs/customs... (accessed 23 May 2013).
LinkOut - more resources
Full Text Sources
Other Literature Sources