A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS
- PMID: 23955302
- PMCID: PMC3789865
- DOI: 10.1038/ncb2822
A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS
Abstract
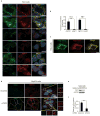
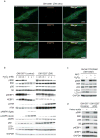
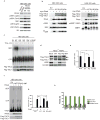
Subcellular localization is emerging as an important mechanism for mTORC1 regulation. We report that the tuberous sclerosis complex (TSC) signalling node, TSC1, TSC2 and Rheb, localizes to peroxisomes, where it regulates mTORC1 in response to reactive oxygen species (ROS). TSC1 and TSC2 were bound by peroxisomal biogenesis factors 19 and 5 (PEX19 and PEX5), respectively, and peroxisome-localized TSC functioned as a Rheb GTPase-activating protein (GAP) to suppress mTORC1 and induce autophagy. Naturally occurring pathogenic mutations in TSC2 decreased PEX5 binding, and abrogated peroxisome localization, Rheb GAP activity and suppression of mTORC1 by ROS. Cells lacking peroxisomes were deficient in mTORC1 repression by ROS, and peroxisome-localization-deficient TSC2 mutants caused polarity defects and formation of multiple axons in neurons. These data identify a role for the TSC in responding to ROS at the peroxisome, and identify the peroxisome as a signalling organelle involved in regulation of mTORC1.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
Figures




Comment in
-
Cell signalling: A peroxisomal home for TSC.Nat Rev Mol Cell Biol. 2013 Oct;14(10):609. doi: 10.1038/nrm3661. Epub 2013 Sep 4. Nat Rev Mol Cell Biol. 2013. PMID: 24002222 No abstract available.
References
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. - PubMed
-
- Gomez MR, Sampson JR, Whittemore VH. Tuberous sclerosis complex. 3. Oxford University Press; New York; Oxford: 1999.
-
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

