Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer
- PMID: 23955427
- PMCID: PMC3816713
- DOI: 10.1002/jso.23392
Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer
Abstract
Background: Trials examining FOLFIRINOX in metastatic pancreatic cancer demonstrate higher response rates compared to gemcitabine-based regimens. There is currently limited experience with neoadjuvant FOLFIRINOX in pancreatic cancer.
Methods: Retrospective review of outcomes of patients with borderline resectable or locally unresectable pancreatic cancer who were recommended to undergo neoadjuvant treatment with FOLFIRINOX.
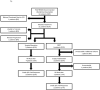
Results: FOLFIRINOX was recommended for 25 patients with pancreatic cancer, 13 (52%) unresectable and 12 (48%) borderline resectable. Four patients (16%) refused treatment or were lost to follow-up. Twenty-one patients (84%) were treated with a median of 4.7 cycles. Six patients (29%) required dose reductions secondary to toxicity. Two patients (9%) were unable to tolerate treatment and three patients (14%) had disease progression on treatment. Seven patients (33%) underwent surgical resection following treatment with FOLFIRINOX alone, 2 (10%) of which were initially unresectable. Two patients underwent resection following FOLFIRINOX + stereotactic body radiation therapy (SBRT). The R0 resection rate for patients treated with FOLFIRINOX ± SBRT was 33% (55% borderline resectable, 10% unresectable). A total of five patients (24%) demonstrated a significant pathologic response.
Conclusions: FOLFIRINOX is a biologically active regimen in borderline resectable and locally unresectable pancreatic cancer with encouraging R0 resection and pathologic response rates.
Keywords: borderline resectable; locally unresectable; neoadjuvant treatment; pancreatic cancer: FOLFIRINOX.
© 2013 Wiley Periodicals, Inc.
Figures

References
-
- Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. discussion 72-63. - PubMed
-
- Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101:587–592. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

