Liposomal resiquimod for the treatment of Leishmania donovani infection
- PMID: 23956375
- PMCID: PMC3861330
- DOI: 10.1093/jac/dkt320
Liposomal resiquimod for the treatment of Leishmania donovani infection
Abstract
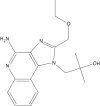
Objectives: The imidazoquinoline family of drugs are Toll-like receptor 7/8 agonists that have previously been used in the treatment of cutaneous leishmaniasis. Because of the hydrophobic nature of imidazoquinolines, they are traditionally not administered systemically for the treatment of visceral leishmaniasis. We formulated liposomal resiquimod, an imidazoquinoline, for the systemic treatment of visceral leishmaniasis.
Methods: By using lipid film hydration with extrusion, we encapsulated resiquimod in liposomes. These liposomes were then injected intravenously to treat BALB/c mice infected with Leishmania donovani.
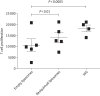
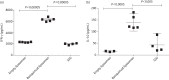
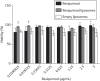
Results: Treatment with liposomal resiquimod significantly decreased the parasite load in the liver, spleen and bone marrow. In addition, resiquimod treatment increased interferon-γ and interleukin-10 production in an antigen recall assay. Resiquimod was shown to be non-toxic in histology and in vitro culture experiments.
Conclusions: FDA-approved resiquimod, in a liposomal formulation, displays promising results in treating visceral leishmaniasis.
Keywords: drug delivery; imidazoquinoline; therapy; visceral.
Figures


Similar articles
-
Combined liposomal immuno- and chemotherapy of visceral leishmaniasis.Arzneimittelforschung. 1999 Nov;49(11):954-61. Arzneimittelforschung. 1999. PMID: 10604050
-
Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis.Mol Pharm. 2013 Mar 4;10(3):1045-55. doi: 10.1021/mp3005098. Epub 2013 Feb 12. Mol Pharm. 2013. PMID: 23320733 Free PMC article.
-
Cationic liposomal sodium stibogluconate (SSG), a potent therapeutic tool for treatment of infection by SSG-sensitive and -resistant Leishmania donovani.Antimicrob Agents Chemother. 2015 Jan;59(1):344-55. doi: 10.1128/AAC.03305-14. Epub 2014 Nov 3. Antimicrob Agents Chemother. 2015. PMID: 25367907 Free PMC article.
-
Mixed formulation of conventional and pegylated liposomes as a novel drug delivery strategy for improved treatment of visceral leishmaniasis.Expert Opin Drug Deliv. 2014 Oct;11(10):1551-60. doi: 10.1517/17425247.2014.932347. Epub 2014 Jun 25. Expert Opin Drug Deliv. 2014. PMID: 24962630
-
Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T cell responses.J Immunol. 2008 Jul 15;181(2):1386-98. doi: 10.4049/jimmunol.181.2.1386. J Immunol. 2008. PMID: 18606693
Cited by
-
High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma.Sci Adv. 2020 Jun 17;6(25):eaba5542. doi: 10.1126/sciadv.aba5542. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32596460 Free PMC article.
-
Polyphosphazene immunoadjuvants: Historical perspective and recent advances.J Control Release. 2021 Jan 10;329:299-315. doi: 10.1016/j.jconrel.2020.12.001. Epub 2020 Dec 5. J Control Release. 2021. PMID: 33285104 Free PMC article. Review.
-
Nano- and Microformulations to Advance Therapies for Visceral Leishmaniasis.ACS Biomater Sci Eng. 2021 May 10;7(5):1725-1741. doi: 10.1021/acsbiomaterials.0c01132. Epub 2020 Oct 14. ACS Biomater Sci Eng. 2021. PMID: 33966377 Free PMC article. Review.
-
The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection.Vaccines (Basel). 2023 Feb 9;11(2):395. doi: 10.3390/vaccines11020395. Vaccines (Basel). 2023. PMID: 36851272 Free PMC article.
-
Liposomal drug delivery systems for the treatment of leishmaniasis.Parasitol Res. 2022 Nov;121(11):3073-3082. doi: 10.1007/s00436-022-07659-5. Epub 2022 Sep 16. Parasitol Res. 2022. PMID: 36112211 Review.
References
-
- Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82. - PubMed
-
- Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. - PubMed
-
- Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–70. - PubMed
-
- Elnaiem DE. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J Vector Ecol. 2011;36(Suppl 1):S23–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

