Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions
- PMID: 23956422
- PMCID: PMC3808972
- DOI: 10.4049/jimmunol.1300342
Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions
Abstract
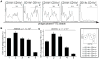
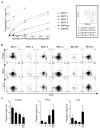
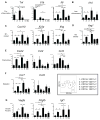
Recent reports have highlighted greater complexity, plasticity, and functional diversity of mononuclear phagocytes (MPCs), including monocytes, macrophages, and dendritic cells (DCs), in our organs than previously understood. The functions and origins of MPCs resident within healthy organs, especially in the kidney, are less well understood, whereas studies suggest they play roles in disease states distinct from recruited monocytes. We developed an unbiased approach using flow cytometry to analyze MPCs residing in the normal mouse kidney, and identified five discrete subpopulations according to CD11b/CD11c expression as well as F4/80, CD103, CD14, CD16, and CD64 expression. In addition to distinct marker profiles, these subpopulations have different lineages and expression of genes involved in tissue homeostasis, including angiogenesis. Among them, the CD11b(int)CD11c(int) F4/80(high) subpopulation notably exhibited high capacity to produce a representative anti-inflammatory cytokine, IL-10. Each subpopulation had different degrees of both macrophage (phagocytosis) and DC (Ag presentation) capacities, with a tendency to promote differentiation of regulatory T cells, whereas two of these showed expression of transcription factors reported to be highly expressed by classical DCs, and proclivity to exit the kidney following stimulation with LPS. In summary, resident kidney MPCs comprise discrete subpopulations, which cannot be simply classified into the conventional entities, and they produce anti-inflammatory and tissue-homeostatic factors to differing degrees.
Figures





References
-
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. - PubMed
-
- del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268–281. - PubMed
Publication types
MeSH terms
Grants and funding
- UH3 TR000504/TR/NCATS NIH HHS/United States
- R01 DK093493/DK/NIDDK NIH HHS/United States
- R37 AI093808/AI/NIAID NIH HHS/United States
- R01 DK083375/DK/NIDDK NIH HHS/United States
- DK84077/DK/NIDDK NIH HHS/United States
- DK93493/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- R24 DK094768/DK/NIDDK NIH HHS/United States
- DK94768/DK/NIDDK NIH HHS/United States
- UH2 TR000504/TR/NCATS NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- TR000504/TR/NCATS NIH HHS/United States
- DK83375/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

