CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-β
- PMID: 23956428
- PMCID: PMC3775269
- DOI: 10.4049/jimmunol.1300830
CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-β
Abstract
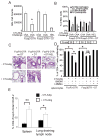
The CD28 costimulatory receptor is a critical regulator of T cell function, making it an attractive therapeutic target for the treatment of immune-mediated diseases. CTLA4Ig, now approved for use in humans, prevents naive T cell activation by binding to B7 proteins and blocking engagement of CD28. However, CTLA4Ig suppresses inflammation even if administered when disease is established, suggesting alternative mechanisms. We identified a novel, CD28-independent mechanism by which CTLA4Ig inhibits activated T cells. We show that in vitro, CTLA4Ig synergizes with NO from bone marrow-derived macrophages to inhibit T cell proliferation. Depletion of regulatory T cells (Tregs) or interference with TGF-β signaling abrogated the inhibitory effect of CTLA4Ig. Parallel in vivo experiments using an allergic airway inflammation model demonstrated that this novel mechanism required both macrophages and regulatory T cells. Furthermore, CTLA4Ig was ineffective in SMAD3-deficient mice, supporting a requirement for TGF-β signaling. Thus, in addition to preventing naive T cells from being fully activated, CTLA4Ig can turn off already activated effector T cells by an NO/regulatory T cell/TGF-β-dependent pathway. This mechanism is similar to cell-extrinsic effects of endogenous CTLA4 and may be particularly important in the ability of CTLA4Ig to treat chronic inflammatory disease.
Conflict of interest statement
There are no conflicts of interest
Figures
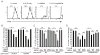

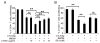
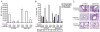

References
-
- Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. - PubMed
-
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. - PubMed
-
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. - PubMed
-
- Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10:411–412. - PubMed
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

