Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering
- PMID: 23956463
- PMCID: PMC3744378
- DOI: 10.1002/macp.201200412
Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering
Abstract
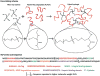
The outstanding elastomeric properties of natural resilin, an insect protein, have motivated the engineering of resilin-like polypeptides (RLPs) as a potential material for cardiovascular tissue engineering. The RLPs, which incorporate biofunctional domains for cell-matrix interactions, are cross-linked into RLP-PEG hybrid hydrogels via a Michael-type addition of cysteine residues on the RLP with vinyl sulfones of an end-functionalized multi-arm star PEG. Oscillatory rheology indicated the useful mechanical properties of these materials. Assessments of cell viability via con-focal microscopy clearly show the successful encapsulation of human aortic adventitial fibroblasts in the three-dimensional matrices and the adoption of a spread morphology following 7 days of culture.
Keywords: biomaterials; elastomers; hydrogels; mechanical properties; tissue engineering.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
