Lipoxin a4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats
- PMID: 23956501
- PMCID: PMC3730367
- DOI: 10.1155/2013/231351
Lipoxin a4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats
Abstract
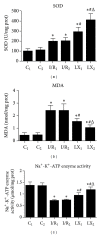
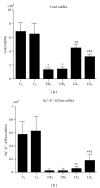
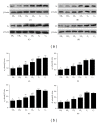
This study aims to investigate the pre- and postconditioning effects of lipoxin A4 (LXA4) on myocardial damage caused by ischemia/reperfusion (I/R) injury. Seventy-two rats were divided into 6 groups: sham groups (C1 and C2), I/R groups (I/R1 and I/R2), and I/R plus LXA4 preconditioning and postconditioning groups (LX1 and LX2). The serum levels of IL-1 β , IL-6, IL-8, IL-10, TNF- α , and cardiac troponin I (cTnI) were measured. The content and the activity of Na(+)-K(+)-ATPase as well as the superoxide dismutase (SOD), and malondialdehyde (MDA) levels were determined. Along with the examination of myocardium ultrastructure and ventricular arrhythmia scores (VAS), connexin 43 (Cx43) expression were also detected. Lower levels of IL-1 β , IL-6, IL-8, TNF- α , cTnI, MDA content, and VAS and higher levels of IL-10, SOD activity, Na(+)-K(+)-ATPase content and activity, and Cx43 expression appeared in LX groups than I/R groups. Besides, H&E staining, TEM examination as well as analysis of gene, and protein confirmed that LXA4 preconditioning was more effective than postconditioning in preventing arrhythmogenesis via the upregulation of Cx43. That is, LXA4 postconditioning had better protective effect on Na(+)-K(+)-ATPase and myocardial ultrastructure.
Figures



References
-
- Hoffman JW, Jr., Gilbert TB, Poston RS, Silldorff EP. Myocardial reperfusion injury: etiology, mechanisms, and therapies. Journal of Extra-Corporeal Technology. 2004;36(4):391–411. - PubMed
-
- Maxwell SRJ, Lip GYH. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. International Journal of Cardiology. 1997;58(2):95–117. - PubMed
-
- Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Annals of Thoracic Surgery. 1999;68(5):1905–1912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

