On the g-protein-coupled receptor heteromers and their allosteric receptor-receptor interactions in the central nervous system: focus on their role in pain modulation
- PMID: 23956775
- PMCID: PMC3730365
- DOI: 10.1155/2013/563716
On the g-protein-coupled receptor heteromers and their allosteric receptor-receptor interactions in the central nervous system: focus on their role in pain modulation
Abstract
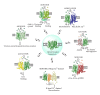
The modulatory role of allosteric receptor-receptor interactions in the pain pathways of the Central Nervous System and the peripheral nociceptors has become of increasing interest. As integrators of nociceptive and antinociceptive wiring and volume transmission signals, with a major role for the opioid receptor heteromers, they likely have an important role in the pain circuits and may be involved in acupuncture. The delta opioid receptor (DOR) exerts an antagonistic allosteric influence on the mu opioid receptor (MOR) function in a MOR-DOR heteromer. This heteromer contributes to morphine-induced tolerance and dependence, since it becomes abundant and develops a reduced G-protein-coupling with reduced signaling mainly operating via β -arrestin2 upon chronic morphine treatment. A DOR antagonist causes a return of the Gi/o binding and coupling to the heteromer and the biological actions of morphine. The gender- and ovarian steroid-dependent recruitment of spinal cord MOR/kappa opioid receptor (KOR) heterodimers enhances antinociceptive functions and if impaired could contribute to chronic pain states in women. MOR1D heterodimerizes with gastrin-releasing peptide receptor (GRPR) in the spinal cord, mediating morphine induced itch. Other mechanism for the antinociceptive actions of acupuncture along meridians may be that it enhances the cross-desensitization of the TRPA1 (chemical nociceptor)-TRPV1 (capsaicin receptor) heteromeric channel complexes within the nociceptor terminals located along these meridians. Selective ionotropic cannabinoids may also produce cross-desensitization of the TRPA1-TRPV1 heteromeric nociceptor channels by being negative allosteric modulators of these channels leading to antinociception and antihyperalgesia.
Figures

Similar articles
-
Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism.PLoS One. 2013;8(3):e58362. doi: 10.1371/journal.pone.0058362. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554887 Free PMC article.
-
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review.
-
Mu and Delta Opioid Receptors Are Coexpressed and Functionally Interact in the Enteric Nervous System of the Mouse Colon.Cell Mol Gastroenterol Hepatol. 2020;9(3):465-483. doi: 10.1016/j.jcmgh.2019.11.006. Epub 2019 Nov 20. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31759144 Free PMC article.
-
Functional reduction in mu-opioidergic system in the spinal cord under a neuropathic pain-like state following chronic ethanol consumption in the rat.Neuroscience. 2007 Feb 9;144(3):777-82. doi: 10.1016/j.neuroscience.2006.10.028. Epub 2006 Dec 6. Neuroscience. 2007. PMID: 17156932
-
Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance.Curr Drug Abuse Rev. 2012 Sep;5(3):199-226. doi: 10.2174/1874473711205030199. Curr Drug Abuse Rev. 2012. PMID: 22920535 Review.
Cited by
-
Emerging Role of (Endo)Cannabinoids in Migraine.Front Pharmacol. 2018 Apr 24;9:420. doi: 10.3389/fphar.2018.00420. eCollection 2018. Front Pharmacol. 2018. PMID: 29740328 Free PMC article. Review.
-
Dopamine D₄ receptor counteracts morphine-induced changes in µ opioid receptor signaling in the striosomes of the rat caudate putamen.Int J Mol Sci. 2014 Jan 21;15(1):1481-98. doi: 10.3390/ijms15011481. Int J Mol Sci. 2014. PMID: 24451133 Free PMC article.
-
Allosteric Modulator Leads Hiding in Plain Site: Developing Peptide and Peptidomimetics as GPCR Allosteric Modulators.Front Chem. 2021 Oct 7;9:671483. doi: 10.3389/fchem.2021.671483. eCollection 2021. Front Chem. 2021. PMID: 34692635 Free PMC article. Review.
-
The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components.Int J Mol Sci. 2014 May 14;15(5):8570-90. doi: 10.3390/ijms15058570. Int J Mol Sci. 2014. PMID: 24830558 Free PMC article.
-
On the role of adenosine (A)₂A receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats.Psychopharmacology (Berl). 2015 Jan;232(2):421-35. doi: 10.1007/s00213-014-3675-2. Epub 2014 Jul 16. Psychopharmacology (Berl). 2015. PMID: 25027583
References
-
- Andersson K, Fuxe K, Hokfelt T, et al. Localization and possible function of peptidergic neurons and their interactions with central catecholamine neurons, and the central actions of gut hormones. Federation Proceedings. 1979;38(9):2333–2340. - PubMed
-
- Agnati LF, Fuxe K, Zini I, Lenzi P, Hokfelt T. Aspects on receptor regulation and isoreceptor identification. Medical Biology. 1980;58(4):182–187. - PubMed
-
- Fuxe K, Agnati LF, Benfenati F, et al. Modulation by cholecystokinins of 3H-spiroperidol binding in rat striatum: evidence for increased affinity and reduction in the number of binding sites. Acta Physiologica Scandinavica. 1981;113(4):567–569. - PubMed
-
- Limbird LE, de Meyts P, Lefkowitz RJ. β Adrenergic receptors: evidence for negative cooperativity. Biochemical and Biophysical Research Communications. 1975;64(4):1160–1168. - PubMed
-
- Fuxe K, Marcellino D, Guidolin D, Woods AS, Agnati L. Brain receptor mosaics and their intramembrane receptor-receptor interactions: molecular integration in transmission and novel targets for drug Development. Journal of Acupuncture and Meridian Studies. 2009;2(1):1–25. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

