Single-Cell Force Spectroscopy of Als-Mediated Fungal Adhesion
- PMID: 23956795
- PMCID: PMC3743104
- DOI: 10.1039/C3AY40473K
Single-Cell Force Spectroscopy of Als-Mediated Fungal Adhesion
Abstract
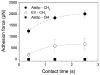
Macroscopic assays that are traditionally used to investigate the adhesion behaviour of microbial cells provide averaged information obtained on large populations of cells and do not measure the fundamental forces driving single-cell adhesion. Here, we use single-cell force spectroscopy (SCFS) to quantify the specific and non-specific forces engaged in the adhesion of the human fungal pathogen Candida albicans. Saccharomyces cerevisiae cells expressing the C. albicans adhesion protein Als5p were attached on atomic force microscopy tipless cantilevers using a bioinspired polydopamine wet polymer, and force-distance curves were recorded between the obtained cell probes and various solid surfaces. Force signatures obtained on hydrophobic substrates exhibited large adhesion forces (1.25 ± 0.2 nN) with extended rupture lengths (up to 400 nm), attributed to the binding and stretching of the hydrophobic tandem repeats of Als5p. Data collected on fibronectin (Fn) -coated substrates featured strong adhesion forces (2.8 ± 0.6 nN), reflecting specific binding between Fn and the N-terminal immunoglobulin-like regions of Als5p, followed by weakly adhesive macromolecular bonds. Both hydrophobic and Fn adhesion forces increased with contact time, emphasizing the important role that time plays in strengthening adhesion. Our SCFS methodology provides a versatile platform in biomedicine for understanding the fundamental forces driving adhesion and biofilm formation in fungal pathogens.
Keywords: Als proteins; Candida albicans; adhesion; atomic force microscopy; force spectroscopy; fungal pathogens; single-cells.
Figures





Similar articles
-
Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction.Nanoscale. 2013 Nov 21;5(22):10894-900. doi: 10.1039/c3nr03272h. Epub 2013 Sep 20. Nanoscale. 2013. PMID: 24057018 Free PMC article.
-
Quantifying the forces driving cell-cell adhesion in a fungal pathogen.Langmuir. 2013 Nov 5;29(44):13473-80. doi: 10.1021/la403237f. Epub 2013 Oct 23. Langmuir. 2013. PMID: 24152214 Free PMC article.
-
Force Sensitivity in Saccharomyces cerevisiae Flocculins.mSphere. 2016 Aug 17;1(4):e00128-16. doi: 10.1128/mSphere.00128-16. eCollection 2016 Jul-Aug. mSphere. 2016. PMID: 27547825 Free PMC article.
-
Sticky microbes: forces in microbial cell adhesion.Trends Microbiol. 2015 Jun;23(6):376-82. doi: 10.1016/j.tim.2015.01.011. Epub 2015 Feb 12. Trends Microbiol. 2015. PMID: 25684261 Review.
-
Statistical analysis of long- and short-range forces involved in bacterial adhesion to substratum surfaces as measured using atomic force microscopy.Appl Environ Microbiol. 2011 Aug;77(15):5065-70. doi: 10.1128/AEM.00502-11. Epub 2011 Jun 3. Appl Environ Microbiol. 2011. PMID: 21642399 Free PMC article. Review.
Cited by
-
The Human Disease-Associated Aβ Amyloid Core Sequence Forms Functional Amyloids in a Fungal Adhesin.mBio. 2016 Jan 12;7(1):e01815-15. doi: 10.1128/mBio.01815-15. mBio. 2016. PMID: 26758179 Free PMC article.
-
Single-Molecule Force Spectroscopy Study on Modular Resilin Fusion Protein.ACS Omega. 2017 Oct 19;2(10):6906-6915. doi: 10.1021/acsomega.7b01133. eCollection 2017 Oct 31. ACS Omega. 2017. PMID: 31457277 Free PMC article.
-
Functional redundancy in Candida auris cell surface adhesins crucial for cell-cell interaction and aggregation.Nat Commun. 2024 Oct 25;15(1):9212. doi: 10.1038/s41467-024-53588-5. Nat Commun. 2024. PMID: 39455573 Free PMC article.
-
Atomic Force Microscopy: A Promising Tool for Deciphering the Pathogenic Mechanisms of Fungi in Cystic Fibrosis.Mycopathologia. 2018 Feb;183(1):291-310. doi: 10.1007/s11046-017-0201-1. Epub 2017 Nov 11. Mycopathologia. 2018. PMID: 29128932 Review.
-
Adhesins of Yeasts: Protein Structure and Interactions.J Fungi (Basel). 2018 Oct 27;4(4):119. doi: 10.3390/jof4040119. J Fungi (Basel). 2018. PMID: 30373267 Free PMC article. Review.
References
-
- Benoit M, Gabriel D, Gerisch G, Gaub HE. Nat Cell Biol. 2000;2(6):313–317. - PubMed
-
- Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Nat Cell Biol. 2008;10(4):429–436. - PubMed
-
- Helenius J, Heisenberg CP, Gaub HE, Muller DJ. J Cell Sci. 2008;121(11):1785–1791. - PubMed
-
- Friedrichs J, Helenius J, Müller DJ. Nat Protoc. 2010;5(7):1353–1361. - PubMed
-
- Bowen WR, Lovitt RW, Wright CJ. J colloid and interface Sci. 2001;237:54–61. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

