Probiotics as additives on therapy in allergic airway diseases: a systematic review of benefits and risks
- PMID: 23956972
- PMCID: PMC3727208
- DOI: 10.1155/2013/231979
Probiotics as additives on therapy in allergic airway diseases: a systematic review of benefits and risks
Abstract
Background: We conducted a systematic review to find out the role of probiotics in treatment of allergic airway diseases.
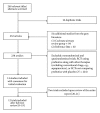
Methods: A comprehensive search of the major electronic databases was done till March 2013. Trials comparing the effect of probiotics versus placebo were included. A predefined set of outcome measures were assessed. Continuous data were expressed as standardized mean difference with 95% CI. Dichotomous data were expressed as odds ratio with 95% CI. P value < 0.05 was considered as significant.
Results: A total of 12 studies were included. Probiotic intake was associated with a significantly improved quality of life score in patients with allergic rhinitis (SMD -1.9 (95% CI -3.62, -0.19); P = 0.03), though there was a high degree of heterogeneity. No improvement in quality of life score was noted in asthmatics. Probiotic intake also improved the following parameters: longer time free from episodes of asthma and rhinitis and decrease in the number of episodes of rhinitis per year. Adverse events were not significant.
Conclusion: As the current evidence was generated from few trials with high degree of heterogeneity, routine use of probiotics as an additive on therapy in subjects with allergic airway diseases cannot be recommended.
Figures


References
-
- Maggi E. The Th1/Th2 paradigm in allergy. Immunotechnology. 1998;3(4):233–244. - PubMed
-
- Akbari O, Stock P, DeKruyff RH, Umetsu DT. Mucosal tolerance and immunity: regulating the development of allergic disease and asthma. International Archives of Allergy and Immunology. 2003;130(2):108–118. - PubMed
-
- Matricardi PM, Bjorksten B, Bonini S, et al. Microbial products in allergy prevention and therapy. Allergy. 2003;58(6):461–471. - PubMed
-
- Heller F, Duchmann R. Intestinal flora and mucosal immune responses. International Journal of Medical Microbiology. 2003;293(1):77–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

