Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes
- PMID: 23957661
- PMCID: PMC3836831
- DOI: 10.1111/cmi.12183
Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes
Abstract
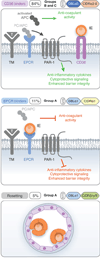
Cytoadhesion of Plasmodium falciparum-infected erythrocytes to host microvasculature is a key virulence determinant. Parasite binding is mediated by a large family of clonally variant adhesion proteins, termed P. falciparum erythrocyte membrane protein 1 (PfEMP1), encoded by var genes and expressed at the infected erythrocyte surface. Although PfEMP1 proteins have extensively diverged under opposing selection pressure to maintain ligand binding while avoiding antibody-mediated detection, recent work has revealed they can be classified into different groups based on chromosome location and domain composition. This grouping reflects functional specialization of PfEMP1 proteins for different human host and microvascular binding niches and appears to be maintained by gene recombination hierarchies. Inone extreme, a specific PfEMP1 variant is associated with placental binding and malaria during pregnancy, while other PfEMP1 subtypes appear to be specialized for infection of malaria naïve hosts. Here, we discuss recent findings on the origins and evolution of the var gene family, the structure-function of PfEMP1 proteins, and a distinct subset of PfEMP1 variants that have been associated with severe childhood malaria.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Baruch DI, Ma XC, Singh HB, Bi X, Pasloske BL, Howard RJ. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. - PubMed
-
- Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

