The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing
- PMID: 23958029
- PMCID: PMC3794711
- DOI: 10.1016/j.biomaterials.2013.08.005
The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing
Abstract
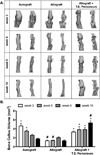
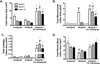
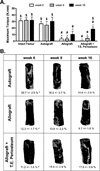
Allografts remain the clinical "gold standard" for treatment of critical sized bone defects despite minimal engraftment and ∼60% long-term failure rates. Therefore, the development of strategies to improve allograft healing and integration are necessary. The periosteum and its associated stem cell population, which are lacking in allografts, coordinate autograft healing. Herein we utilized hydrolytically degradable hydrogels to transplant and localize mesenchymal stem cells (MSCs) to allograft surfaces, creating a periosteum mimetic, termed a 'tissue engineered periosteum'. Our results demonstrated that this tissue engineering approach resulted in increased graft vascularization (∼2.4-fold), endochondral bone formation (∼2.8-fold), and biomechanical strength (1.8-fold), as compared to untreated allografts, over 16 weeks of healing. Despite this enhancement in healing, the process of endochondral ossification was delayed compared to autografts, requiring further modifications for this approach to be clinically acceptable. However, this bottom-up biomaterials approach, the engineered periosteum, can be augmented with alternative cell types, matrix cues, growth factors, and/or other small molecule drugs to expedite the process of ossification.
Keywords: Bone allografts; Hydrogels; Mesenchymal stem cells; Periosteum; Regenerative medicine; Tissue engineering.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing.Biomaterials. 2015 Jun;52:426-40. doi: 10.1016/j.biomaterials.2015.02.064. Epub 2015 Mar 18. Biomaterials. 2015. PMID: 25818449 Free PMC article.
-
Emerging ideas: Engineering the periosteum: revitalizing allografts by mimicking autograft healing.Clin Orthop Relat Res. 2013 Mar;471(3):721-6. doi: 10.1007/s11999-012-2695-7. Epub 2012 Nov 21. Clin Orthop Relat Res. 2013. PMID: 23179118 Free PMC article.
-
Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature.Biomaterials. 2021 Jan;268:120535. doi: 10.1016/j.biomaterials.2020.120535. Epub 2020 Nov 19. Biomaterials. 2021. PMID: 33271450 Free PMC article.
-
A perspective: engineering periosteum for structural bone graft healing.Clin Orthop Relat Res. 2008 Aug;466(8):1777-87. doi: 10.1007/s11999-008-0312-6. Epub 2008 May 29. Clin Orthop Relat Res. 2008. PMID: 18509709 Free PMC article. Review.
-
Potential mechanisms of a periosteum patch as an effective and favourable approach to enhance tendon-bone healing in the human body.Int Orthop. 2012 Mar;36(3):665-9. doi: 10.1007/s00264-011-1346-z. Epub 2011 Oct 19. Int Orthop. 2012. PMID: 22009448 Free PMC article. Review.
Cited by
-
Non-invasive diffuse correlation tomography reveals spatial and temporal blood flow differences in murine bone grafting approaches.Biomed Opt Express. 2016 Aug 9;7(9):3262-3279. doi: 10.1364/BOE.7.003262. eCollection 2016 Sep 1. Biomed Opt Express. 2016. PMID: 27699097 Free PMC article.
-
Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction.Biomaterials. 2018 Nov;182:279-288. doi: 10.1016/j.biomaterials.2018.08.028. Epub 2018 Aug 14. Biomaterials. 2018. PMID: 30142527 Free PMC article.
-
Temporal blood flow changes measured by diffuse correlation tomography predict murine femoral graft healing.PLoS One. 2018 May 29;13(5):e0197031. doi: 10.1371/journal.pone.0197031. eCollection 2018. PLoS One. 2018. PMID: 29813078 Free PMC article.
-
Construction of biomimetic cell-sheet-engineered periosteum with a double cell sheet to repair calvarial defects of rats.J Orthop Translat. 2022 Oct 14;38:1-11. doi: 10.1016/j.jot.2022.09.005. eCollection 2023 Jan. J Orthop Translat. 2022. PMID: 36313975 Free PMC article.
-
Periosteum Containing Implicit Stem Cells: A Progressive Source of Inspiration for Bone Tissue Regeneration.Int J Mol Sci. 2024 Feb 10;25(4):2162. doi: 10.3390/ijms25042162. Int J Mol Sci. 2024. PMID: 38396834 Free PMC article. Review.
References
-
- Farfalli GL, Aponte-Tinao L, Lopez-Millan L, Ayerza MA, Muscolo DL. Clinical and functional outcomes of tibial intercalary allografts after tumor resection. Orthopedics. 2012;35(3):e391–e396. - PubMed
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. - PubMed
-
- Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A(Pt 2) Suppl 2:98–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

