Evaluating a nurse-led survivorship care package (SurvivorCare) for bowel cancer survivors: study protocol for a randomized controlled trial
- PMID: 23958184
- PMCID: PMC3765148
- DOI: 10.1186/1745-6215-14-260
Evaluating a nurse-led survivorship care package (SurvivorCare) for bowel cancer survivors: study protocol for a randomized controlled trial
Abstract
Background: Colorectal cancer (CRC) is the most common cancer affecting both men and women in Australia. The illness and related treatments can cause distressing adverse effects, impact on emotional and psychological well-being, and adversely affect social, occupational and relationship functioning for many years after the end of treatment or, in fact, lifelong. Current models of follow-up fail to address the complex needs arising after treatment completion. Strategies to better prepare and support survivors are urgently required. We previously developed a nurse-led supportive care program (SurvivorCare) and tested it in a pilot study involving 10 CRC survivors. The intervention was found to be highly acceptable, appropriate, relevant and useful.
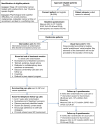
Methods/design: This study is a multisite, randomised controlled trial, designed to assess the impact of the addition of the SurvivorCare intervention to usual post-treatment care, for people with potentially cured CRC. SurvivorCare comprises the provision of survivorship educational materials, a tailored survivorship care plan, an individually tailored nurse-led, face-to-face end of treatment consultation and three subsequent telephone calls. Eligible patients have completed treatment for potentially cured CRC. Other eligibility criteria include stage I to III disease, age greater than 18 years and adequate understanding of English. All consenting patients complete questionnaires at three time points over a six-month period (baseline, two and six months). Measures assess psychological distress, unmet needs and quality of life.
Discussion: This supportive care package has the potential to significantly reduce individual suffering, whilst reducing the burden of follow-up on acute cancer services through enhanced engagement with and utilisation of general practitioners and community based services. If the intervention is successful in achieving the expected health benefits, it could be disseminated readily. All training and supporting materials have been developed and standardised. Furthermore, the intervention could easily be adapted to other cancer or chronic disease settings.
Trial registration: Australian New Zealand Clinical Trial Registry ACTRN12610000207011.
Figures
References
-
- McDermid I, AIHW, AACR, NCSG. Cancer Incidence Projections Australia 2002 to 2011. Canberra, ACT: Australian Institute of Health and Welfare (AIHW), Australasian Association of Cancer Registries (AACR) and the National Cancer Strategies Group (NCSG); 2005.
-
- Edna T-H, Bjerkeset T. Small bowel obstruction in patients previously operated on for colorectal cancer. Eur J Surg. 1998;164:587–592. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical