Reversibility of regorafenib effects in hepatocellular carcinoma cells
- PMID: 23959464
- PMCID: PMC3836575
- DOI: 10.1007/s00280-013-2269-8
Reversibility of regorafenib effects in hepatocellular carcinoma cells
Abstract
Purpose: Multikinase growth inhibitors inhibit their target kinases with varying potency. Patients often require lower doses or therapy breaks due to drug toxicities. To evaluate the effects of drug withdrawal on hepatocellular carcinoma cells after incubation with growth-inhibitory concentrations of regorafenib, cell growth, migration and invasion, and signaling were examined.
Methods: Cell proliferation, motility, and invasion were analyzed by MTT, wound healing, and invasion assays, respectively, and MAPK pathway protein markers were analyzed by Western blot.
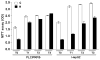
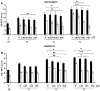
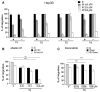
Results: After regorafenib removal, cell growth, migration, and invasion recovered. Repeated drug exposure resulted in changes in cell growth patterns. Recovery could be blocked by sub-growth-inhibitory concentrations of either doxorubicin or vitamin K1. Recovery of growth was associated with increased phospho-JNK, phospho-p38, and phospho-STAT3 levels. The recovery of growth, migration, and signaling were blocked by a JNK inhibitor.
Conclusions: Removal of regorafenib from growth-inhibited cells resulted in a JNK-dependent recovery of growth and migration.
Figures



References
-
- Ahmad T, Eisen T. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res. 2004;10:6388S–6392S. - PubMed
-
- Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843–2855. - PubMed
-
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. - PubMed
-
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

