Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis
- PMID: 23959575
- PMCID: PMC4139146
- DOI: 10.1002/hep.26697
Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis
Abstract
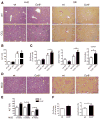
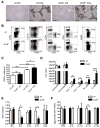
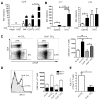
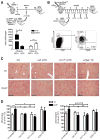
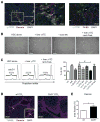
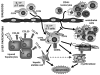
Chronic liver injury promotes hepatic inflammation, representing a prerequisite for organ fibrosis. We hypothesized a contribution of chemokine receptor CCR6 and its ligand, CCL20, which may regulate migration of T-helper (Th)17, regulatory, and gamma-delta (γδ) T cells. CCR6 and CCL20 expression was intrahepatically up-regulated in patients with chronic liver diseases (n = 50), compared to control liver (n = 5). Immunohistochemistry revealed the periportal accumulation of CCR6(+) mononuclear cells and CCL20 induction by hepatic parenchymal cells in liver disease patients. Similarly, in murine livers, CCR6 was expressed by macrophages, CD4 and γδ T-cells, and up-regulated in fibrosis, whereas primary hepatocytes induced CCL20 upon experimental injury. In two murine models of chronic liver injury (CCl4 and methionine-choline-deficient diet), Ccr6(-/-) mice developed more severe fibrosis with strongly enhanced hepatic immune cell infiltration, compared to wild-type (WT) mice. Although CCR6 did not affect hepatic Th-cell subtype composition, CCR6 was explicitly required by the subset of interleukin (IL)-17- and IL-22-expressing γδ T cells for accumulation in injured liver. The adoptive transfer of WT γδ, but not CD4 T cells, into Ccr6(-/-) mice reduced hepatic inflammation and fibrosis in chronic injury to WT level. The anti-inflammatory function of hepatic γδ T cells was independent of IL-17, as evidenced by transfer of Il-17(-/-) cells. Instead, hepatic γδ T cells colocalized with hepatic stellate cells (HSCs) in vivo and promoted apoptosis of primary murine HSCs in a cell-cell contact-dependent manner, involving Fas-ligand (CD95L). Consistent with γδ T-cell-induced HSC apoptosis, activated myofibroblasts were more frequent in fibrotic livers of Ccr6(-/-) than in WT mice.
Conclusion: γδ T cells are recruited to the liver by CCR6 upon chronic injury and protect the liver from excessive inflammation and fibrosis by inhibiting HSCs.
© 2013 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

References
-
- Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets. 2011;10:509–536. - PubMed
-
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. - PubMed
-
- Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
