Potential roles of microglial cell progranulin in HIV-associated CNS pathologies and neurocognitive impairment
- PMID: 23959579
- PMCID: PMC3930627
- DOI: 10.1007/s11481-013-9495-z
Potential roles of microglial cell progranulin in HIV-associated CNS pathologies and neurocognitive impairment
Abstract
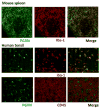
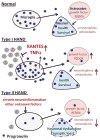
Progranulin (PGRN) is a highly unusual molecule with both neuronal and microglial expression with two seemingly unrelated functions, i.e., as a neuronal growth factor and a modulator of neuroinflammation. Haploinsufficiency due to loss of function mutations lead to a fatal presenile dementing illness (frontotemporal lobar degeneration), indicating that adequate expression of PGRN is essential for successful aging. PGRN might be a particularly relevant factor in the pathogenesis of HIVencephalitis (HIVE) and HIV-associated neurocognitive disorders (HAND). We present emerging data and a review of the literature which show that cells of myeloid lineage such as macrophages and microglia are the primary sources of PGRN and that PGRN expression contributes to pathogenesis of CNS diseases. We also present evidence that PGRN is a macrophage antiviral cytokine. For example, PGRN mRNA and protein expression are significantly upregulated in brain specimens with HIVE, and in HIV infected microglia in vitro. Paradoxically, our preliminary CHARTER data analyses indicate that lower PGRN levels in CSF trended towards an association with HAND, particularly in those without detectable virus. Based upon these findings, we introduce the hypothesis that PGRN plays dual roles in modulating antiviral immunity and neuronal dysfunction in the context of HIV infection. In the presence of active viral replication, PGRN expression is increased functioning as an anti-viral factor as well as a neuroprotectant. In the absence of active HIV replication, ongoing inflammation or other stressors suppress PGRN production from macrophages/microglia contributing to neurocognitive dysfunction. We propose.
Conflict of interest statement
Figures




References
-
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. - PMC - PubMed
-
- Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukoc Biol. 1995;58:643–649. - PubMed
-
- Arkins S, Rebeiz N, Brunke-Reese DL, Biragyn A, Kelley KW. Interferon-gamma inhibits macrophage insulin-like growth factor-I synthesis at the transcriptional level. Mol Endocrinol. 1995;9:350–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01MH083500/MH/NIMH NIH HHS/United States
- HHSN271201000030C/MH/NIMH NIH HHS/United States
- U01 MH083501/MH/NIMH NIH HHS/United States
- U01 MH083506/MH/NIMH NIH HHS/United States
- U24 MH100930/MH/NIMH NIH HHS/United States
- U01 MH083507/MH/NIMH NIH HHS/United States
- KO1MH084705/MH/NIMH NIH HHS/United States
- R01MH55477/MH/NIMH NIH HHS/United States
- U01 MH083500/MH/NIMH NIH HHS/United States
- P30AI051519/AI/NIAID NIH HHS/United States
- U24 MH100928/MH/NIMH NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- HHSN271201000036C/MH/NIMH NIH HHS/United States
- R01 MH055477/MH/NIMH NIH HHS/United States
- K01 MH084705/MH/NIMH NIH HHS/United States
- U01MH083501/MH/NIMH NIH HHS/United States
- U01MH083506/MH/NIMH NIH HHS/United States
- U01 MH083545/MH/NIMH NIH HHS/United States
- N01 MH022005/MH/NIMH NIH HHS/United States
- U01MH083507/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

