Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis
- PMID: 23959857
- PMCID: PMC3824388
- DOI: 10.1158/0008-5472.CAN-13-0926
Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis
Abstract
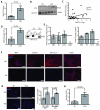
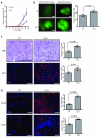
Obesity is one of the most important preventable causes of cancer and the most significant risk factor for breast cancer in postmenopausal women. Compared with lean women, obese women are more likely to be diagnosed with a larger, higher grade tumor, an increased incidence of lymph node metastases, and elevated risk of distant recurrence. However, the mechanisms connecting obesity to the pathogenesis of breast cancer are poorly defined. Here, we show that during obesity, adipocytes within human and mouse breast tissues recruit and activate macrophages through a previously uncharacterized CCL2/IL-1β/CXCL12 signaling pathway. Activated macrophages in turn promote stromal vascularization and angiogenesis even before the formation of cancer. Recapitulating these changes using a novel humanized breast cancer model was sufficient to promote angiogenesis and prime the microenvironment prior to neoplastic transformation for accelerated breast oncogenesis. These findings provide a mechanistic role for adipocytes and macrophages before carcinogenesis that may be critical for prevention and treatment of obesity-related cancer.
©2013 AACR.
Figures




References
-
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. - PubMed
-
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. - PubMed
-
- Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006;113:1160–6. - PubMed
-
- Brown KA, Simpson ER. Obesity and breast cancer: mechanisms and therapeutic implications. Front Biosci (Elite Ed) 2012;4:2515–24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

