Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance
- PMID: 23959870
- PMCID: PMC3767519
- DOI: 10.1073/pnas.1302212110
Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance
Abstract
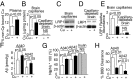
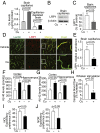
Whereas amyloid-β (Aβ) accumulates in the brain of normal animals dosed with low levels of copper (Cu), the mechanism is not completely known. Cu could contribute to Aβ accumulation by altering its clearance and/or its production. Because Cu homeostasis is altered in transgenic mice overexpressing Aβ precursor protein (APP), the objective of this study was to elucidate the mechanism of Cu-induced Aβ accumulation in brains of normal mice and then to explore Cu's effects in a mouse model of Alzheimer's disease. In aging mice, accumulation of Cu in brain capillaries was associated with its reduction in low-density lipoprotein receptor-related protein 1 (LRP1), an Aβ transporter, and higher brain Aβ levels. These effects were reproduced by chronic dosing with low levels of Cu via drinking water without changes in Aβ synthesis or degradation. In human brain endothelial cells, Cu, at its normal labile levels, caused LRP1-specific down-regulation by inducing its nitrotyrosination and subsequent proteosomal-dependent degradation due in part to Cu/cellular prion protein/LRP1 interaction. In APP(sw/0) mice, Cu not only down-regulated LRP1 in brain capillaries but also increased Aβ production and neuroinflammation because Cu accumulated in brain capillaries and, unlike in control mice, in the parenchyma. Thus, we have demonstrated that Cu's effect on brain Aβ homeostasis depends on whether it is accumulated in the capillaries or in the parenchyma. These findings should provide unique insights into preventative and/or therapeutic approaches to control neurotoxic Aβ levels in the aging brain.
Keywords: BACE1; BBB; cerebrovascular; environmental; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




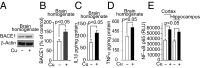
References
-
- Lutsenko S, Bhattacharjee A, Hubbard AL (2010) Copper handling machinery of the brain. Metallomics 2(9):596–608. - PubMed
-
- Chutkow JG (1978) Evidence for uptake of nonceruloplasminic copper in the brain: Effect of ionic copper and amino acids. Proc Soc Exp Biol Med. 158(1):113–116. - PubMed
-
- Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. - PubMed
-
- Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors. 2012;38(2):107–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

