Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome
- PMID: 23959892
- PMCID: PMC3767560
- DOI: 10.1073/pnas.1300600110
Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome
Abstract
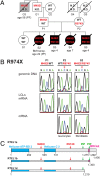
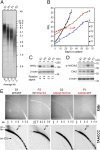
Telomeres repress the DNA damage response at the natural chromosome ends to prevent cell-cycle arrest and maintain genome stability. Telomeres are elongated by telomerase in a tightly regulated manner to ensure a sufficient number of cell divisions throughout life, yet prevent unlimited cell division and cancer development. Hoyeraal-Hreidarsson syndrome (HHS) is characterized by accelerated telomere shortening and a broad range of pathologies, including bone marrow failure, immunodeficiency, and developmental defects. HHS-causing mutations have previously been found in telomerase and the shelterin component telomeric repeat binding factor 1 (TRF1)-interacting nuclear factor 2 (TIN2). We identified by whole-genome exome sequencing compound heterozygous mutations in four siblings affected with HHS, in the gene encoding the regulator of telomere elongation helicase 1 (RTEL1). Rtel1 was identified in mouse by its genetic association with telomere length. However, its mechanism of action and whether it regulates telomere length in human remained unknown. Lymphoblastoid cell lines obtained from a patient and from the healthy parents carrying heterozygous RTEL1 mutations displayed telomere shortening, fragility and fusion, and growth defects in culture. Ectopic expression of WT RTEL1 suppressed the telomere shortening and growth defect, confirming the causal role of the RTEL1 mutations in HHS and demonstrating the essential function of human RTEL1 in telomere protection and elongation. Finally, we show that human RTEL1 interacts with the shelterin protein TRF1, providing a potential recruitment mechanism of RTEL1 to telomeres.
Keywords: aging; dyskeratosis congenita; genomic instability; telomeropathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jain D, Cooper JP. Telomeric strategies: Means to an end. Annu Rev Genet. 2010;44:243–269. - PubMed
-
- de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. - PubMed
-
- Liu D, O’Connor MS, Qin J, Songyang Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J Biol Chem. 2004;279(49):51338–51342. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

