Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation
- PMID: 23959899
- PMCID: PMC3767499
- DOI: 10.1073/pnas.1313945110
Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation
Abstract
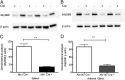
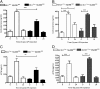
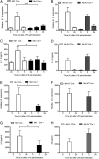
Inflammation-related dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is central to the course of systemic inflammatory response syndrome or sepsis. The underlying mechanisms, however, are not well understood. Initial activation of adrenocortical hormone production during early sepsis depends on the stimulation of hypothalamus and pituitary mediated by cytokines; in late sepsis, there is a shift from neuroendocrine to local immune-adrenal regulation of glucocorticoid production. Therefore, the modulation of the local immune-adrenal cross talk, and not of the neuroendocrine circuits involved in adrenocorticotropic hormone production, may be more promising in the prevention of the adrenal insufficiency associated with prolonged sepsis. In the present work, we investigated the function of the crucial Toll-like receptor (TLR) adaptor protein myeloid differentiation factor 88 (MyD88) in systemic and local activation of adrenal gland inflammation and glucocorticoid production mediated by lipopolysachharides (LPSs). To this end, we used mice with a conditional MyD88 allele. These mice either were interbred with Mx1 Cre mice, resulting in systemic MyD88 deletion, predominantly in the liver and hematopoietic system, or were crossed with Akr1b7 Cre transgenic mice, resulting thereby in deletion of MyD88, which was adrenocortical-specific. Although reduced adrenal inflammation and HPA-axis activation mediated by LPS were found in Mx1(Cre+)-MyD88(fl/fl) mice, adrenocortical-specific MyD88 deletion did not alter the adrenal inflammation or HPA-axis activity under systemic inflammatory response syndrome conditions. Thus, our data suggest an important role of immune cell rather than adrenocortical MyD88 for adrenal inflammation and HPA-axis activation mediated by LPS.
Keywords: Toll-like receptors; adrenal gland insufficiency; the HPA axis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29(7) Suppl:S109–S116. - PubMed
-
- Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360(22):2328–2339. - PubMed
-
- Annane D, et al. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174(12):1319–1326. - PubMed
-
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. - PubMed
-
- Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13(5):480–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

