Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells
- PMID: 23959938
- PMCID: PMC3800758
- DOI: 10.1210/en.2013-1242
Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells
Abstract
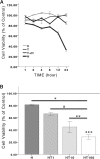
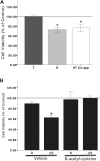
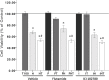
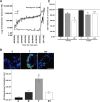
Males have a higher risk for developing Parkinson's disease and parkinsonism after ischemic stroke than females. Although estrogens have been shown to play a neuroprotective role in Parkinson's disease, there is little information on androgens' actions on dopamine neurons. In this study, we examined the effects of androgens under conditions of oxidative stress to determine whether androgens play a neuroprotective or neurotoxic role in dopamine neuronal function. Mitochondrial function, cell viability, intracellular calcium levels, and mitochondrial calcium influx were examined in response to androgens under both nonoxidative and oxidative stress conditions. Briefly, N27 dopaminergic cells were exposed to the oxidative stressor, hydrogen peroxide, and physiologically relevant levels of testosterone or dihydrotestosterone, applied either before or after oxidative stress exposure. Androgens, alone, increased mitochondrial function via a calcium-dependent mechanism. Androgen pretreatment protected cells from oxidative stress-induced cell death. However, treatment with androgens after the oxidative insult increased cell death, and these effects were, in part, mediated by calcium influx into the mitochondria. Interestingly, the negative effects of androgens were not blocked by either androgen or estrogen receptor antagonists. Instead, a putative membrane-associated androgen receptor was implicated. Overall, our results indicate that androgens are neuroprotective when oxidative stress levels are minimal, but when oxidative stress levels are elevated, androgens exacerbate oxidative stress damage.
Figures

Similar articles
-
Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment.Biol Sex Differ. 2020 Mar 29;11(1):12. doi: 10.1186/s13293-020-0283-1. Biol Sex Differ. 2020. PMID: 32223745 Free PMC article.
-
NADPH Oxidase Mediates Membrane Androgen Receptor-Induced Neurodegeneration.Endocrinology. 2019 Apr 1;160(4):947-963. doi: 10.1210/en.2018-01079. Endocrinology. 2019. PMID: 30811529 Free PMC article.
-
Effects of Oxidative Stress and Testosterone on Pro-Inflammatory Signaling in a Female Rat Dopaminergic Neuronal Cell Line.Endocrinology. 2016 Jul;157(7):2824-35. doi: 10.1210/en.2015-1738. Epub 2016 May 11. Endocrinology. 2016. PMID: 27167771 Free PMC article.
-
The influence of pharmaceutical compounds on male fertility.Andrologia. 1976;8(3):203-35. doi: 10.1111/j.1439-0272.1976.tb02137.x. Andrologia. 1976. PMID: 793446 Review.
-
Androgens and Parkinson's Disease: A Review of Human Studies and Animal Models.Androg Clin Res Ther. 2021 Dec 23;2(1):294-303. doi: 10.1089/andro.2021.0011. eCollection 2021. Androg Clin Res Ther. 2021. PMID: 35024696 Free PMC article. Review.
Cited by
-
Re-Cloning the N27 Dopamine Cell Line to Improve a Cell Culture Model of Parkinson's Disease.PLoS One. 2016 Aug 11;11(8):e0160847. doi: 10.1371/journal.pone.0160847. eCollection 2016. PLoS One. 2016. PMID: 27512998 Free PMC article.
-
Epigenetic Regulation of Oxidative Stress in Ischemic Stroke.Aging Dis. 2016 May 27;7(3):295-306. doi: 10.14336/AD.2015.1009. eCollection 2016 May. Aging Dis. 2016. PMID: 27330844 Free PMC article. Review.
-
Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment.Biol Sex Differ. 2020 Mar 29;11(1):12. doi: 10.1186/s13293-020-0283-1. Biol Sex Differ. 2020. PMID: 32223745 Free PMC article.
-
The association of vitamin D status with oxidative stress biomarkers and matrix metalloproteinases in patients with knee osteoarthritis.Front Nutr. 2023 Feb 8;10:1101516. doi: 10.3389/fnut.2023.1101516. eCollection 2023. Front Nutr. 2023. PMID: 36845046 Free PMC article.
-
Ligand-free mitochondria-localized mutant AR-induced cytotoxicity in spinal bulbar muscular atrophy.Brain. 2023 Jan 5;146(1):278-294. doi: 10.1093/brain/awac269. Brain. 2023. PMID: 35867854 Free PMC article.
References
-
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134 - PubMed
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909 - PubMed
-
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55(3):259–272 - PubMed
-
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302(5646):819–822 - PubMed
-
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104(3):431–449 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

