Primary marrow-derived stromal cells: isolation and manipulation
- PMID: 23959984
- PMCID: PMC3748384
- DOI: 10.1007/978-1-62703-508-8_8
Primary marrow-derived stromal cells: isolation and manipulation
Abstract
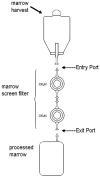
Marrow stromal cells (MSCs) are relatively rare cells difficult to visualize in marrow biopsies or detect in aspirated marrow. Under specific conditions MSC can be expanded in vitro and the population can give rise to several mesenchymal lineages. "MSC" also refers to mesenchymal stem cells which implies that all cells in the population are multipotent. It is generally agreed that while there may be a few multipotent stem cells in an MSC population the majority are not stem cells. In either case MSCs do not produce hematopoietic cells. Although MSCs have been isolated and characterized from several tissues, bone marrow is their most common source for research and clinical use. Primary MSC populations can be derived from bone marrow mononuclear cells with relative ease, but it is important to recognize the cellular heterogeneity within a culture and how this may vary from donor to donor. In this chapter, we describe methodology to derive primary MSCs from bone marrow screens, an otherwise discarded by-product of bone marrow harvests used for clinical transplantation. We also describe some useful techniques to characterize and manipulate MSCs-both primary and immortalized cell lines.
Figures






References
-
- Chabannon C, Torok-Storb B. Stem cell-stromal cell interactions. Curr Top Microbiol Immunol. 1992;177:123–36. - PubMed
-
- McCulloch EA, Siminovitch L, Till JE, Russell ES, Bernstein SE. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype sl-sld. Blood. 1965;26:399–410. - PubMed
-
- Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, et al. The hematopoietic growth factor KL is encoded by the sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–33. - PubMed
-
- Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL094374/HL/NHLBI NIH HHS/United States
- R01 HL104070/HL/NHLBI NIH HHS/United States
- HL104070/HL/NHLBI NIH HHS/United States
- HL099993/HL/NHLBI NIH HHS/United States
- R03 DK082757/DK/NIDDK NIH HHS/United States
- K08 DK073701/DK/NIDDK NIH HHS/United States
- DK082757/DK/NIDDK NIH HHS/United States
- P30 DK056465/DK/NIDDK NIH HHS/United States
- DK073701/DK/NIDDK NIH HHS/United States
- U01 HL099993/HL/NHLBI NIH HHS/United States
- K08 DK082783/DK/NIDDK NIH HHS/United States
- DK082783/DK/NIDDK NIH HHS/United States
- DK056465/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

