Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1
- PMID: 23960033
- PMCID: PMC3844542
- DOI: 10.1213/ANE.0b013e3182a00ae0
Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1
Abstract
Background: We previously demonstrated that propofol interacted with the leukocyte adhesion molecule leukocyte function-associated antigen-1 (LFA-1) and inhibited the production of interleukin-2 via LFA-1 in a dependent manner. However, the binding site(s) of propofol on LFA-1 remains unknown.
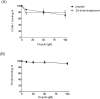
Methods: First, the inhibition of LFA-1's ligand binding by propofol was confirmed in an enzyme-linked immunosorbent assay (ELISA) ELISA-type assay. The binding site of propofol on LFA-1 was probed with a photolabeling experiment using a photoactivatable propofol analog called azi-propofol-m. The adducted residues of LFA-1 by this compound were determined using liquid chromatography-mass spectrometry. In addition, the binding of propofol to the ligand-binding domain of LFA-1 was examined using 1-aminoanthracene (1-AMA) displacement assay. Furthermore, the binding site(s) of 1-AMA and propofol on LFA-1 was studied using the docking program GLIDE.
Results: We demonstrated that propofol impaired the binding of LFA-1 to its ligand intercellular adhesion molecule-1. The photolabeling experiment demonstrated that the adducted residues were localized in the allosteric cavity of the ligand-binding domain of LFA-1 called "lovastatin site." The shift of fluorescence spectra was observed when 1-AMA was coincubated with the low-affinity conformer of LFA-1 ligand-binding domain (wild-type [WT] αL I domain), not with the high-affinity conformer, suggesting that 1-AMA bound only to WT αL I domain. In the 1-AMA displacement assay, propofol decreased 1-AMA fluorescence signal (at 520 nm), suggesting that propofol competed with 1-AMA and bound to the WT αL I domain. The docking simulation demonstrated that both 1-AMA and propofol bound to the lovastatin site, which agreed with the photolabeling experiment.
Conclusions: We demonstrated that propofol bound to the lovastatin site in LFA-1. Previously we showed that the volatile anesthetics isoflurane and sevoflurane bound to this site. Taken together, the lovastatin site is an example of the common binding sites for anesthetics currently used clinically.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
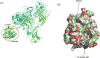
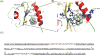

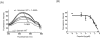
Similar articles
-
Stereoselectivity of isoflurane in adhesion molecule leukocyte function-associated antigen-1.PLoS One. 2014 May 6;9(5):e96649. doi: 10.1371/journal.pone.0096649. eCollection 2014. PLoS One. 2014. PMID: 24801074 Free PMC article.
-
Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1.Anesthesiology. 2010 Sep;113(3):600-9. doi: 10.1097/ALN.0b013e3181e89a77. Anesthesiology. 2010. PMID: 20693879 Free PMC article.
-
Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin αIIbβ3.PLoS One. 2013;8(4):e60415. doi: 10.1371/journal.pone.0060415. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573252 Free PMC article.
-
Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1.FASEB J. 2012 Nov;26(11):4408-17. doi: 10.1096/fj.12-212746. Epub 2012 Jul 19. FASEB J. 2012. PMID: 22815384 Free PMC article.
-
Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen.J Immunotoxicol. 2016;13(2):148-56. doi: 10.3109/1547691X.2015.1019596. Epub 2015 Mar 9. J Immunotoxicol. 2016. PMID: 25746395 Free PMC article.
Cited by
-
Shedding Light on Anesthetic Mechanisms: Application of Photoaffinity Ligands.Anesth Analg. 2016 Nov;123(5):1253-1262. doi: 10.1213/ANE.0000000000001365. Anesth Analg. 2016. PMID: 27464974 Free PMC article. Review.
-
Risk factors for pediatric surgical site infection following neurosurgical procedures for hydrocephalus: a retrospective single-center cohort study.BMC Anesthesiol. 2021 Apr 21;21(1):124. doi: 10.1186/s12871-021-01342-5. BMC Anesthesiol. 2021. PMID: 33882858 Free PMC article.
-
In Vitro Model of Stretch-Induced Lung Injury to Study Different Lung Ventilation Regimens and the Role of Sedatives.Transl Perioper Pain Med. 2020;7(3):258-264. Epub 2020 May 15. Transl Perioper Pain Med. 2020. PMID: 32542183 Free PMC article.
-
Perioperative and long-term outcomes of spontaneous ventilation video-assisted thoracoscopic surgery for non-small cell lung cancer.Transl Lung Cancer Res. 2021 Oct;10(10):3875-3887. doi: 10.21037/tlcr-21-629. Transl Lung Cancer Res. 2021. PMID: 34858778 Free PMC article.
-
The Role of Anesthetic Selection in Perioperative Bleeding.Biomed Res Int. 2021 May 7;2021:5510634. doi: 10.1155/2021/5510634. eCollection 2021. Biomed Res Int. 2021. PMID: 34036098 Free PMC article. Review.
References
-
- Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25:551–70. ix. - PubMed
-
- Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77. - PubMed
-
- Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–43. - PubMed
-
- Von Dossow V, Baur S, Sander M, Tønnesen H, Marks C, Paschen C, Berger G, Spies CD. Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J Int Med Res. 2007;35:395–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

